Loading metrics
Open Access
Peer-reviewed
Research Article

Relating Structure and Function in the Human Brain: Relative Contributions of Anatomy, Stationary Dynamics, and Non-stationarities
* E-mail: [email protected]
Affiliation Laboratoire d'Imagerie Fonctionnelle, UMR678, Inserm/UPMC Univ Paris 06, Paris, France
- Arnaud Messé,
- David Rudrauf,
- Habib Benali,
- Guillaume Marrelec

- Published: March 20, 2014
- https://doi.org/10.1371/journal.pcbi.1003530
- Reader Comments
Investigating the relationship between brain structure and function is a central endeavor for neuroscience research. Yet, the mechanisms shaping this relationship largely remain to be elucidated and are highly debated. In particular, the existence and relative contributions of anatomical constraints and dynamical physiological mechanisms of different types remain to be established. We addressed this issue by systematically comparing functional connectivity (FC) from resting-state functional magnetic resonance imaging data with simulations from increasingly complex computational models, and by manipulating anatomical connectivity obtained from fiber tractography based on diffusion-weighted imaging. We hypothesized that FC reflects the interplay of at least three types of components: (i) a backbone of anatomical connectivity, (ii) a stationary dynamical regime directly driven by the underlying anatomy, and (iii) other stationary and non-stationary dynamics not directly related to the anatomy. We showed that anatomical connectivity alone accounts for up to 15% of FC variance; that there is a stationary regime accounting for up to an additional 20% of variance and that this regime can be associated to a stationary FC; that a simple stationary model of FC better explains FC than more complex models; and that there is a large remaining variance (around 65%), which must contain the non-stationarities of FC evidenced in the literature. We also show that homotopic connections across cerebral hemispheres, which are typically improperly estimated, play a strong role in shaping all aspects of FC, notably indirect connections and the topographic organization of brain networks.
Author Summary
By analogy with the road network, the human brain is defined both by its anatomy (the ‘roads’), that is, the way neurons are shaped, clustered together and connected to each others and its dynamics (the ‘traffic’): electrical and chemical signals of various types, shapes and strength constantly propagate through the brain to support its sensorimotor and cognitive functions, its capacity to learn and adapt to disease, and to create consciousness. While anatomy and dynamics are organically intertwined (anatomy contributes to shape dynamics), the nature and strength of this relation remain largely mysterious. Various hypotheses have been proposed and tested using modern neuroimaging techniques combined with mathematical models of brain activity. In this study, we demonstrate the existence (and quantify the contribution) of a dynamical regime in the brain, coined ‘stationary’, that appears to be largely induced and shaped by the underlying anatomy. We also reveal the critical importance of specific anatomical connections in shaping the global anatomo-functional structure of this dynamical regime, notably connections between hemispheres.
Citation: Messé A, Rudrauf D, Benali H, Marrelec G (2014) Relating Structure and Function in the Human Brain: Relative Contributions of Anatomy, Stationary Dynamics, and Non-stationarities. PLoS Comput Biol 10(3): e1003530. https://doi.org/10.1371/journal.pcbi.1003530
Editor: Claus C. Hilgetag, Hamburg University, Germany
Received: August 14, 2013; Accepted: February 8, 2014; Published: March 20, 2014
Copyright: © 2014 Messé et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work is supported by the Inserm and the University Pierre et Marie Curie (Paris, France). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Coherent behavior and cognition involve synergies between neuronal populations in interaction [1] – [3] . Even at rest, in the absence of direct environmental stimulations, these interactions drive the synchronization of spontaneous activity across brain systems, shedding light on the large-scale anatomo-functional organization of the brain [4] . The study of such patterns of synchronization has known important developments due to recent methodological advances in brain imaging data acquisition and analysis. These advances have enabled investigators to estimate interactions in the brain by measuring functional connectivity (FC) from resting-state functional MRI (rs-fMRI). Analyses of FC at rest have supported the hypothesis that the brain is spatially organized into large-scale intrinsic networks [5] – [7] , e.g. the so-called resting-state networks [8] , [9] , such as the default mode network, which have been linked to central integrative cognitive functions [10] – [13] . The study of large-scale intrinsic networks from rs-fMRI has become a central and active area for neuroscience research. However, the mechanisms and factors driving FC, as well as their relative contribution to empirical data, are still highly debated [14] and remain to be elucidated.
Theoretical rationale and empirical findings support the hypothesis that FC is driven and shaped by structural connectivity (SC) between brain systems, i.e., by the actual bundles of white matter fiber connecting neurons [15] . As a first approximation, SC can be inferred from fiber tractography based on diffusion-weighted imaging (DWI) [16] – [19] . A recent study [20] , which focused on a small subset of robustly estimated structural connections, demonstrated the existence of a statistical, yet complex, correspondence between FC and specific features of SC (e.g., low vs. high fiber density, short vs. long fibers, intra vs. interhemispheric connections). However, a large part of FC cannot be explained by SC alone [21] . There appears that FC is the result of at least two main contributing factors: (i) the underlying anatomical structure of connectivity, and (ii) the dynamics of neuronal populations emerging from their physiology [3] . A key issue is to better understand the relative contributions of these two components to FC. Besides, recent studies using windowed analyses have suggested that FC estimated over an entire acquisition session (referred to as ‘stationary FC’ in the literature) breaks down into a variety of reliable correlation patterns (also referred to as ‘dynamic FC’ or ‘non-stationarities’) when estimated over short time windows (30 s) [14] , [22] . The authors advocated that FC estimated over short time windows (or windowed FC, for short) mostly reflects recurrent transitory patterns that are aggregated when estimating FC over a whole session. They further suggested that whole-session FC may only be an epiphenomenon without clear physiological underpinning, and not the reflection of an actual process with stationary FC [14] . This perspective remains to be reconciled with the fact that whole-session FC has been found to be highly reproducible, functionally meaningful and a useful biomarker in many pathological contexts [23] , [24] . Note that, in the recent literature of fMRI data analysis, stationarity implicitely refers to a stationary FC (i.e., the invariance of FC over time), to be contrasted with the more general notion of (strong) stationarity, where a model or process is stationary if its parameters remain constant over time [25] , [26] . SC being temporally stable at the scale of a whole resting state fMRI session (typically 10 min), we could expect SC to drive a stationary process (in the strong sense). Since SC is furthermore expected to drive FC, we can hypothesize that this stationary process contributes to generate a stationary FC.
In order to bring together the structural and dynamical components underlying FC, some studies have used computational models that incorporate SC together with biophysical models of neuronal activity to generate coherent brain dynamics [27] – [32] . This approach has yielded promising results for the understanding of the relationship between structure and function [17] , [33] , [34] . Here, we used a testbed of well-established generative models simulating neuronal dynamics combined with empirical measures, to investigate the relative contributions of anatomical connections, stationary dynamics, and non-stationarities to the emergence of empirical functional connectivity. In particular, we considered the following hypotheses: (H1) part of FC directly reflects SC; (H2) models of physiological mechanisms added to SC increase predictive power all the more as they are complex; (H3) part of the variance of FC that is unexplained by models is due to issues in the estimation of SC, e.g., problems with measuring homotopic connections; (H4) there is an actual stationary process reflected in whole-session FC that is not merely an artifact but substantially reflects the driving of the dynamics by SC.
In order to test these hypotheses and estimate the relative contribution of anatomy, stationary dynamics and non-stationarities to FC, we relied on the following approach. After T 1 -weighted MRI based parcellation of the brain ( N = 160 regions), SC was estimated using the proportion of white matter fibers connecting pairs of regions, based on probabilistic tractography of DWI data [35] . FC was measured on rs-fMRI data using Pearson correlation between the time courses of brain regions. We quantified the correlation between SC alone and FC as a reference, and also fed SC to generative neurodynamical models of increasing complexity: a spatial autoregressive (SAR) model [36] , analytic models with or without conduction delays [28] – [31] , [37] , and biologically constrained models [29] , [32] . Importantly, all these models were used in their stationary regime in the strong sense, since their parameters were not changed during the simulations. Of these models, only the SAR is explicitely associated with a stationary FC; other, more complex models, generate dynamics that are compatible with a non-stationary FC. We computed FC from data simulated by these models and compared the results to empirical FC. For each model, performance was quantified using predictive power [29] , for each subject as well as on the ‘average subject’ (obtained by averaging SC and empirical FC across subjects). Values for the model parameters were based on the literature, except for the structural coupling strength that was optimized in order to maximize each model's performance.
Predictive power of models
In agreement with H1, SC explained a significant amount of the variance of whole-session FC for all subjects, as did all generative models (permutation test, p <0.05 corrected) ( Figure 1 , panel A). Generative models predicted FC better than SC alone (paired permutation test, p <0.05 corrected). Predictive power obtained with the average subject ranged from 0.32 for SC alone to 0.43 for the SAR model ( Table 1 ). For a given model, predictive power was reproducible across subjects. Contrary to our hypothesis H2, generative models had similar performance, and complexity was not predictive of performance. The results remained unchanged when no global signal regression was applied ( Figure S1 ). Also, findings were found to be similar for SC alone and the SAR model at finer spatial scales ( N = 461 and N = 825 regions, Figure S2 ) and consistent with a replication dataset ( Figure S3 ). Most importantly, a large part of the variance ( R 2 ) in the empirical data (at least 82%) remained unexplained by this first round of simulations.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
(A) Predictive power for all connections and when restricted to intra/interhemispheric, direct/indirect connections. For each type of connections and each model, we represented the individual predictive powers (bar chart representing means and associated standard deviations), as well as the predictive power for the average subject computed using the original SC (diamonds), or after adding homotopic connections (circles). Of note, SC alone has no predictive power (zero) for the subset of indirect connections, by definition. (B) Patterns of SC, empirical FC and FC simulated from the SAR model for the average subject and associated scatter plot of simulated versus empirical FC (solid line represents perfect match). SARh stands for the SAR model with added homotopic connections. Matrices were rearranged such that network structure is highlighted. Homologous regions were arranged symmetrically with respect to the center of the matrix; for instance, the first and last regions are homologous. (C) Similarity of functional brain networks across subjects (bar chart with means and associated standard deviations), for the average subject (diamonds), and when adding homotopic connections (circles) (left). Network maps for the average subject and empirical FC, as well as for FC simulated using either the SAR model with original SC or the SARh.
https://doi.org/10.1371/journal.pcbi.1003530.g001
https://doi.org/10.1371/journal.pcbi.1003530.t001
Role of homotopic connections
We reasoned (see hypothesis H3) that part of the unexplained variance could reflect issues with the estimation of SC from DWI, which can be expected because of limitations in current fiber tracking algorithms and the problem of crossing fibers [38] . We know for instance that many fibers passing through the corpus callosum are poorly estimated in diffusion imaging, in particular those connecting more lateral parts of the cerebral cortex [39] . Yet, the corpus callosum is the main interhemispheric commissure of the mammal brain, see [40] . It systematically connects homologous sectors of the cerebral cortex across the two hemispheres in a topographically organized manner, with an antero-posterior gradient, through a system of myelinated homotopic fibers or ‘homotopic connections’. The hypothesis of an impact of SC estimation problems on FC unexplained variance was supported by the observation that, in our results, intrahemispheric connections yielded on average a much higher predictive power (e.g., 0.59 for the SAR model) than interhemispheric connections (0.16 for the SAR model).
In order to further test the role of white matter connections in driving FC, we artificially set all homotopic connections to a constant SC value (0.5) for the average subject and reran all simulations. As a result, the predictive power strongly increased for all models ( Figure 1 , panels A and B), ranging from 0.39 for SC alone to 0.61 for the SAR model ( Table 1 ). Thus the variance unexplained (1- R 2 ) was reduced to 63%. Moreover, predictive power for intra and interhemispheric connections became equivalent (0.60 and 0.62, respectively). Interestingly, adding homotopic connections also led to a substantial increase in predictive power for indirect connections, that is, pairs of regions for which SC is zero (increasing from 0.07 to 0.45). The effect of adding interhemispheric anatomical connections on increasing predictive power was highly specific to homotopic connections. When applying the SAR model to the SC matrix with added homotopic connections and randomly permuting (10 000 permutations) the 80 corresponding interhemispheric connections (one region in one hemisphere was connected to one and only one region in the other hemisphere), the predictive power strongly decreased, even compared to results with the original SC ( Figure 2 , panel A). Moreover, we further assessed the specificity of this result by systematically manipulating SC. In three different simulations, we randomly removed, added, and permuted structural connections (10 000 times). In all cases, the predictive power decreased as a function of the proportion of connections manipulated ( Figure 2 , panel B). Moreover, changes induced by these manipulations remained small (<0.05), far below the changes that we were able to induce by adding homotopic connections. All in all, these results suggest that homotopic connections play a key role in shaping the network dynamics, in a complex and non-trivial manner.
(A) Predictive power of the SAR model with original SC (green), when adding homotopic connections (‘SARh’, red), or with shuffled homotopic connections (black). (B) Predictive power of the SAR model with original SC (red) and when SC values were randomly permuted, removed or added (from left to right). For each graph, predictive power was quantified as a function of the percentage of connections manipulated.
https://doi.org/10.1371/journal.pcbi.1003530.g002
Predicting functional brain networks
Beyond predicting the overall pattern of FC, we also assessed whether models could predict the empirical organization of FC into a set of intrinsic networks. Connectivity matrices were clustered into groups of non-overlapping brain regions showing high within-group correlation and low between-group correlation, and the resulting partitions into functional brain networks were compared between empirical and simulated FC using the adjusted Rand index (see Methods ). Again, the SAR model tended to perform best among all computational models ( Figure 1 , panel C).
Without adding homotopic connections in the SC matrix, the simulated networks highly differed from the empirical networks. In particular, most networks were found to be lateralized. After adding homotopic connections, the resemblence between simulated and empirical networks greatly improved. Networks were more often bilateral and overall consistent with the topography of empirical functional networks, including somatosensory, motor, visual, and associative networks. High FC between the amygdala and ventral-lateral sectors of the prefrontal cortex was also correctly predicted by the simulations. There were nevertheless some notable differences. First, the clustering of empirical FC yielded a long-range fronto-parieto-temporal association network ( Figure 1 , panel C, cyan) that was not observed in simulated FC as such. Second, a parieto-temporal cluster ( Figure 1 , panel C, red), which was associated with thalamo-striatal networks, was predicted by simulations but was not present in the empirical data. Third, a cluster encompassing the entire cingulate gyrus and precuneus ( Figure 1 , panel C, green) was predicted by simulations but was broken down into more clusters in the empirical data.
Stationary FC, non-stationary FC, and non-stationarities
The results above show that SC plays a causal role in FC, but one can still wonder what aspects of the underlying dynamics are the most directly related to this influence. A hypothesis is that SC, in combination with stable physiological processes (e.g., overall gain in synaptic transmission), drives a stationary regime of the dynamics. This hypothesis is supported by the finding that all models tested in this study, which were used in a stationary regime (in the strong sense), could explain significantly more variance than SC alone. Furthermore, the fact that the SAR could predict FC significantly better than all other models is evidence that this stationary regime is associated with stationary FC (paired permutation test, p <0.05 corrected).
But, clearly, many variations in the dynamical patterns of brain activity, be it in the process of spontaneous cognition, physiological regulation, or context-dependent changes, cannot be expected to be associated with a purely stationary FC. Modeling how the brain dynamics deal with endogenous and environmental contexts should require more complex models, either stationary or non-stationary, that are able to generate non-stationary (i.e., time-varying) patterns of FC. Given that at best 37% of the variance could be explained by the model of a purely stationary FC (the SAR), we can wonder why the models of higher complexity used in our simulation testbed did not perform better in predicting FC. One possible hypothesis is that the SAR model was favored in the simulations, because we estimated FC over about 10 minutes of actual brain dynamics. In such configuration, we can imagine that the non-stationarities of FC cancel out, the estimation effectively keeping the stationary part of FC. We thus wondered whether the more complex models would better perform when non-stationary FC had the potential of being more strongly reflected in the data. We approached this question by computing predictive power on windowed FC as a function of the length of the time-window used [22] , for all possible time-windows over which FC could be estimated and for all models. We also investigated the effect of simulation duration (see Methods ). We found that the relative performance of more complex models was still lower than that of the SAR model ( Figures 3 and S4 ). The average predictive power was lower for shorter time-windows and increased towards a limit for longer time-windows. The SAR model behaved like an ‘upper-bound’ for predictive power. The performance of all other models, irrespective of the size of the time-window, was between that of SC alone and that of the SAR model.
Predictive power as a function of time-window length across subjects (left) and of duration of simulated runs on the average subject (right). For color code see Figure 1 .
https://doi.org/10.1371/journal.pcbi.1003530.g003
A straightforward explanation is that the non-stationary patterns of FC, as generated by the simulation models, did not match the non-stationary patterns of the empirical FC as they unfolded during the acquisition in the brain of the participants. Context-dependent and transient dynamics are likely to be missed by models of the dynamics that cannot be contextually constrained in the absence of further information. It is thus difficult to infer how much of the 63% of unexplained variance remaining in whole-session FC actually reflect physiologically meaningful non-stationary FC, and more broadly, non-stationary dynamics.
In the present study, we investigated the respective contributions of anatomical connections, stationary dynamics, and non-stationarities to the emergence of empirical functional connectivity. We compared the performance of computational models in modeling FC and manipulated SC in order to analyze the impact of SC on FC, with and without the filter of combined physiological models of the dynamics.
The importance of white matter fiber pathways in shaping functional brain networks is a known fact, for a review, see [15] , [17] , [21] , [23] . Previous modeling studies have supported the importance of the underlying anatomical connections, i.e., SC, in shaping functional relationships among brain systems [16] , [41] , [42] . In agreement with our hypothesis H1, we showed that functional connectivity could at least in part be explained by structural connectivity alone. Adding homotopic connections in the matrix of SC, we found a slight increase in explained variance when considering the prediction of whole-session FC from SC alone (+4% of explained variance). In agreement with H2, adding models of physiological interactions above and beyond SC alone increased the explained variance in whole-session FC, by 8% for the best performing model, the SAR model, when no homotopic connections were added, and by 22% when homotopic connections were added. This latter fact, which strongly supports H3, suggests a complex interplay between anatomy as reflected by SC and physiological mechanisms in generating FC. This impact of SC manipulations on predicted FC pertained not only to direct but also to indirect connections. For indirect connections, whole-session FC was much better predicted after adding homotopic connections to SC than before adding them (0.45 versus 0.07 in predictive power). The problem of limited predictive power for FC based on SC when considering indirect connections has puzzled the field [43] . For this reasons many studies only assess the performance of models on direct connections. Here, we showed that a major factor in driving FC for indirect anatomical connections (+20% in explained variance) is the interplay between a subset of anatomical connections, i.e., homotopic connections (which are typically underestimated by DWI), and physiological parameters that generate the dynamics underlying FC, themselves conditioned by the possible interactions defined by SC.
Contrary to our expectation (see hypothesis H2), all models tended to perform similarly, irrespective of model complexity. The best performing model in most cases was the SAR model, a model of stationary FC driven by SC, with 63% of the variance remaining unexplained. It is likely that, above and beyond problems with the estimation of SC from DWI, and other incompressible sources of irrelevant noise, much of the unexplained variance in FC relates to non-stationary patterns in FC, and more generally to non-stationarities in the strong-sense. Such non-stationarities are difficult to model in the experimentally unconstrained resting-state and in the absence of further information regarding the specific parameters shaping FC. Irrespective of their complexity, computational models are only capable of generating prototypical brain activities, and not the subject-dependent activity that took place in the brain of the participants during scanning. The scientific necessity of modeling brain dynamics is hindered by such uncertainty and it will be a challenge to find solutions to approach this problem [26] , [44] . Even though one objective for neuroscience is to propose generative models that are capable of generating detailed neuronal dynamics, generative models cannot be informed by this unknown context and, as a consequence, cannot generate context-dependent activity in a manner that would be predictive of empirical data, in the absence of additional measures and experimental controls. Nevertheless, and perhaps for that very reason, the study of non-stationarity in FC should become of central interest for the field, as such non-stationarities could explain much of FC (up to 63% according to our simulation results), and thus reflect critical mechanisms for neurocognitive processing.
In the absence of adequate modeling principles, determining the precise contribution of non-stationarities to the unexplained variance in FC is impossible, as other confounding sources of unexplained variance are expected. As we showed, even naive manipulations aimed at estimating the impact of the known errors in DWI-based reconstruction of homotopic connections showed that such errors could cause 20% of the unexplained variance in predicting empirical FC. How DWI and fiber tracking should be used for an optimal estimation of structural connectivity is still a topic of intense debates [45] – [48] . It is likely that part of the unexplained variance in predicting FC will be reduced as better estimates of SC become available.
The model showing the best results, the SAR model, explicitly modeled a stationary process with a stationary FC. In line with our hypothesis H4, empirical FC is likely to incorporate stationary components driven by SC. Further knowledge about this stationary process might be gained by analyzing FC computed over much longer periods of time than is commonly performed (e.g., hours versus minutes). This stationary process is itself likely to be only locally stationary, as it might be expected that slow physiological cycles, from nycthemeral cycles to hormonal cycles, development, learning and aging, will modify the parameters controlling it.
In the present study, we did not take into account the statistical fluctuations induced by the fact that the time series were of finite length. Such a finiteness entails that even a model that is stationary in the strong sense could generate sample moments that fluctuate over time. For instance, the sample sum of square of a multivariate normal model with covariance matrix Σ computed from time series of size N is not equal to N Σ but is Wishart distributed with N -1 degrees of freedom and scale matrix Σ. This phenomenon will artificially increase the part of variance that cannot be accounted for by stationary models and, hence, play against stationary models. Since it is conversely very unlikely for a non-stationary model to generate sample moments that are constant over time, statistical fluctuations cannot at the same time artificially increase the part of variance that can be accounted for by stationary models. As a consequence, not considering these statistical fluctuations made us underestimate the part of variance that can be accounted for by models that are stationary in the strong sense. In other words, our estimate of the part of variance accounted for by a stationary model is a lower bound for the true value. We can therefore be confident that taking statistical fluctuations into account will only strengthen H4.
Our goal here was to investigate how current generative models of brain acticity fare in predicting the relationship between structure and function. The complexity of some of these models was such that the simulations included here were only possible thanks to a computer cluster. The behavior of all these models depends on the values of some parameters and, in the present study, we set these parameters in agreement with the literature. In what measure this choice affects how well models predict FC is unclear. Yet a full investigation of this issue remains beyond the scope of this study, since parameter optimization through extensive exploration of the parameter space for all models is at this stage unrealistic. Nevertheless, in order to get a sense of the sensitivity of our results to parameter values in a way that is compatible with the computational power available, we explored the behavior of the Fitzhugh-Nagumo, Wilson and Kuramoto models over a subset of the parameter space (see Figures S5 and S6 ). We found that parameter values had little influence on predictive power, which, in all cases, remained below that of the SAR, the simplest model tested.
We formulated H2 to test for the existence of a relationship between complexity and realism in the models that we used. Indeed, there should exist a very tight connection between the two, since the more complex generative models in our study have been designed to take biophysical mechanisms into account, with parameters that are physiologically relevant and values often chosen based on prior experimental results. Now, realism usually comes at the cost of complexity. As a consequence, it is often (implicitely) assumed that, among the models we selected, the more complex a model is, the more realistic it will also be and the better it will fit the data. This is the reason why we stated H2, based on such rationale inspired from the literature, in order to put such hypothesis to the test. The results show that for the models we used, with their sets of parameters, an increase in complexity was not associated with an increase in performance. This suggests that, for these models, complexity and realism are not quite as tightly connected as expected.
Given that the SAR model is the only model that does not include a step of hemodynamic modeling (Balloon-Windkessel), it cannot be ruled out that the superiority of the SAR reflects issues with this step. In order to check that this is not the case, we computed predictive power for all models without the hemodynamic model. The predictive power was largely insensitive to the presence of the hemodynamic model (see Figure S7 ). In particular, the SAR model remained overall an upper bound in terms of predictive power.
Finally, we should note that we relied on a definition of SC restricted to the white matter compartment. Although this is standard in the field, in reality, local intrinsic SC exists in the gray matter. However, current models generally make prior assumptions about such SC. Moreover, intrinsic SC currently remains impossible to measure reliably for the entire brain.
In spite of the complexity of the problems and the limitations of current modeling approaches, computational modeling of large-scale brain dynamics remains an essential scientific endeavor. It is key to better understand generative mechanisms and make progress in brain physiology, physiopathology and, more generally, theoretical neuroscience. It is also central to the endeavor of searching for accurate and meaningful biomarkers in aging and disease [49] . Moreover, computational modeling of FC opens the possibility of making inference on specific biophysical parameters, including inference about the underlying anatomical connectivity itself. In spite of their limited predictive powers, simpler models can be useful in this context. The SAR model, introduced in [36] , may appear well-suited to model essential stationary aspects of the generative mechanisms of FC. One interest of such a simple and analytically tractable model is that, beyond its very low computational burden, it could be the basis for straightforward estimation of the model parameters that can be used to compare clinical populations, and could constitute a potentially important biomarker of disease.
Ethics statement
All participants gave written informed consent and the protocol was approved by the local Ethics Committee of the Pitié-Salpêtrière Hospital (number: 44-08; Paris, France).
Twenty-one right-handed healthy volunteers were recruited within local community (11 males, mean age 22±2.4 years). Data were acquired using a 3 T Siemens Trio TIM MRI scanner (CENIR, Paris, France). For acquisition and preprocessing details, see Text S1 . For each subject, the preprocessing yielded three matrices: one of SC, one with the average fiber lengths, and one of empirical FC. These matrices were also averaged across subjects (‘average subject’).
Simulations
We used eight generative models with various levels of complexity: the SAR model, a purely spatial model with no dynamics that expresses BOLD fluctuations within one region as a linear combination of the fluctuations in other regions; the Wilson-Cowan system, a model expressing excitatory and inhibitory neuronal populations activity; the two rate models (with or without conduction delays), simplified versions of the Wilson-Cowan system obtained by considering exclusively the excitatory population; the Kuramoto model, which simulates neuronal activity using oscillators; the Fitzhugh-Nagumo model, which aims at reproducing complex behaviors such as those observed in conductance-based models; the neural-mass model, also based on conductance and with strong biophysiological constraints; and finally, the model of spiking neurons, the most constrained model in the current study which models neuron populations as attractors. For more details, see Text S2 .
All models took an SC matrix as input, and all but the SAR were taken as models of neuronal (rather than BOLD) activity. Simulated fMRI BOLD signal was obtained from simulated neuronal activity by means of the Balloon-Windkessel hemodynamic model [50] , [51] . Global mean signal was then regressed out from each region's time series. Finally, simulated FC was computed as Pearson correlation between simulated time series. For the SAR model, we directly computed simulated FC from the analytical expression of the covariance, see Equation (2) in Text S2 . All models had a parameter that represents the coupling strength between regions. This parameter was optimized separately for each model on the average subject to limit computational burden ( Text S2 ). After optimization, we generated three runs of 8 min BOLD activity and averaged the corresponding FCs to obtain the simulated FC for each dynamical model and each subject. For the average subject, simulated FC was obtained by feeding the average SC matrix to the different models.
Performance
Modeling performance was assessed using predictive power and similarity of spatial patterns. Predictive power was quantified for each subject and for the average subject by means of Pearson correlation between simulated and empirical FC [29] . Regarding the similarity of functional brain networks, SC, empirical FC and simulated FC were decomposed into 10 networks using agglomerative hierarchical clustering and generalized Ward criterion [52] . The resulting networks from SC and simulated FC were compared to the ones resulting from empirical FC using the adjusted Rand index [53] , [54] . The Rand index quantifies the similarity between two partitions of the brain into networks by computing the proportion of pairs of regions for which the two partitions are consistent (i.e., they are either in the same network for both partitions, or in a different network for both partitions). The adjustment accounts for the level of similarity that would be expected by chance only.
Analysis of dynamics
Empirical and simulated windowed FC were computed on individual subjects using sliding time-windows (increment of 20 s) of varying length (from 20 to 420 s by step of 20 s). Predictive power was computed as the correlation between any pair of time-windows of equal length corresponding to simulated and empirical windowed FC, respectively. This approach was only applied to the dynamical models; for SC alone and the SAR model, simulated FC remained, by definition, constant through time and, as a consequence, windowed FC was equaled to whole-session FC. The influence of simulated run duration on predictive power was also investigated. For each model, three runs of one hour were simulated on the average subject. Predictive power was then computed as a function of simulated run duration. For the same reason as above, SC alone and the SAR model did not depend on simulation duration.
Supporting Information
Performance of computational models when no global signal regression was performed. (A) Predictive power for all connections and when restricted to intra/interhemispheric, direct/indirect connections. For each type of connections and each model, we represented the individual predictive powers (bar chart representing means and associated standard deviations), as well as the predictive power for the average subject computed using the original SC (diamonds), or after adding homotopic connections (circles). Of note, SC alone has no predictive power (zero) for the subset of indirect connections, by definition. (B) Patterns of SC, empirical FC and FC simulated from the SAR model for the average subject and associated scatter plot of simulated versus empirical FC (solid line represents perfect match). SARh stands for the SAR model with added homotopic connections. Matrices were rearranged such that network structure is highlighted. Homologous regions were arranged symmetrically with respect to the center of the matrix; for instance, the first and last regions are homologous. (C) Similarity of functional brain networks across subjects (bar chart with means and associated standard deviations), for the average subject (diamonds), and when adding homotopic connections (circles) (left). Network maps for the average subject and empirical FC, as well as for FC simulated using either the SAR model with original SC or the SARh.
https://doi.org/10.1371/journal.pcbi.1003530.s001
Performance of SC alone and the SAR model at finer spatial scales. Predictive power for all connections and when restricted to intra/interhemispheric, direct/indirect connections. For each type of connections and each model, we represented the individual predictive powers (bar chart representing mean and associated standard deviation), as well as the predictive power of the average subject computed using the original SC (diamonds), or after adding homotopic connections (circles).
https://doi.org/10.1371/journal.pcbi.1003530.s002
Performance of computational models on the replication dataset. The replication dataset was from the study of Hagmann and colleagues [55] . Brain network was defined at low anatomical granularity (N = 66 regions), and connectivity measures were averaged over five healthy volunteer subjects. (A) Predictive power for all connections and when restricted to intra/interhemispheric, direct/indirect connections. For each type of connections and each model, we represented the individual predictive powers (bar chart representing means and associated standard deviations), as well as the predictive power for the average subject computed using the original SC (diamonds), or after adding homotopic connections (circles). Of note, SC alone has no predictive power (zero) for the subset of indirect connections, by definition. (B) Patterns of SC, empirical FC and FC simulated from the SAR model for the average subject and associated scatter plot of simulated versus empirical FC (solid line represents perfect match). SARh stands for the SAR model with added homotopic connections. Matrices were rearranged such that network structure is highlighted. Homologous regions were arranged symmetrically with respect to the center of the matrix; for instance, the first and last regions are homologous. (C) Similarity of functional brain networks across subjects (bar chart with means and associated standard deviations), for the average subject (diamonds), and when adding homotopic connections (circles) (left). Network maps for the average subject and empirical FC, as well as for FC simulated using either the SAR model with original SC or the SARh.
https://doi.org/10.1371/journal.pcbi.1003530.s003
Effect of time on performance. Predictive power of computational models as a function of the time-window length for each subject (graphs) and model (color).
https://doi.org/10.1371/journal.pcbi.1003530.s004
Exploration of the parameter space for the Fitzhugh-Nagumo model. (Left) Phase diagrams (i.e., x - y plane) for an uncoupled model ( k = 0) over various parameter values of α and β . The model operate mostly in an oscillatory regime for the range of parameter values investigated. (Right) Predictive power as a function of α and β . The black dot represents the parameter set used in our simulations, while the black square corresponds to the values from [28] . The values used in our simulations gave rise to higher predictive power than the parameters values from [28] . In any case, for the range of parameters considered, the predictive power always remained lower than that obtained with a SAR model.
https://doi.org/10.1371/journal.pcbi.1003530.s005
Effect of velocity on predictive power. Predictive power as a function of the coupling strength and velocity values in generative models. Black dots represent values used for subsequent simulations. These simulations show that the predictive power is little influenced by velocity. In any case, for the range of parameters considered, the predictive power also always remained lower than that obtained with a SAR model.
https://doi.org/10.1371/journal.pcbi.1003530.s006
Effect of the hemodynamic model. Predictive power for all connections and when restricted to intra/interhemispheric, direct/indirect connections. For each type of connections and each model, we represented the predictive power for the average subject computed using the BOLD signal (diamonds, solid line) or using the neuronal activity (circles, dashed line). Of note, the prediction differs slightly from that of the Figure 1 due to the stochastic component of most models at each run.
https://doi.org/10.1371/journal.pcbi.1003530.s007
Data and preprocessing.
https://doi.org/10.1371/journal.pcbi.1003530.s008
Computational models.
https://doi.org/10.1371/journal.pcbi.1003530.s009
Acknowledgments
The authors are thankful to Olaf Sporns (Department of Psychology, Princeton University, Princeton, USA) and Christopher J. Honey (Department of Psychological and Brain Sciences, Indiana University, Bloomington, USA) for providing the neural-mass model; to Gustavo Deco, Étienne Hugues and Joanna Cabral (Computational Neuroscience Group, Department of Technology, Universitat pompeu Fabra, Barcelona, Spain) for providing the Kuramoto and rate models as well as the spike model; and to Olaf Sporns and Patric Hagmann (Department of Radiology, University Hospital Center and University of Lausanne, Lausanne, Switzerland) for sharing their data for replication. We would also like to thank them for fruitful discussions. The authors are grateful to Stéphane Lehéricy and his team (Center for Neuroimaging Research, Paris, France) for providing them with the data, and especially to Romain Valabrègue for his help in handling coarse-grained distributed parallelization of computational tasks.
Author Contributions
Conceived and designed the experiments: AM DR HB GM. Performed the experiments: AM DR GM. Analyzed the data: AM. Wrote the paper: AM DR HB GM.
- View Article
- Google Scholar
- 40. Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press.
- 52. Batageli V (1988) Generalized ward and related clustering problems. In: Bock HH, editor. Classification and Related Methods of Data Analysis. North-Holland. pp. 67–74.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.

Journey to a key front in climate-change fight

A birder’s biggest enemy in rainforest: complacency

Redefining the good life
Epic science inside a cubic millimeter of brain.

Six layers of excitatory neurons color-coded by depth.
Credit: Google Research and Lichtman Lab
Anne J. Manning
Harvard Staff Writer
Researchers publish largest-ever dataset of neural connections
A cubic millimeter of brain tissue may not sound like much. But considering that that tiny square contains 57,000 cells, 230 millimeters of blood vessels, and 150 million synapses, all amounting to 1,400 terabytes of data, Harvard and Google researchers have just accomplished something stupendous.
Led by Jeff Lichtman, the Jeremy R. Knowles Professor of Molecular and Cellular Biology and newly appointed dean of science , the Harvard team helped create the largest 3D brain reconstruction to date, showing in vivid detail each cell and its web of connections in a piece of temporal cortex about half the size of a rice grain.
Published in Science, the study is the latest development in a nearly 10-year collaboration with scientists at Google Research, combining Lichtman’s electron microscopy imaging with AI algorithms to color-code and reconstruct the extremely complex wiring of mammal brains. The paper’s three first co-authors are former Harvard postdoc Alexander Shapson-Coe, Michał Januszewski of Google Research, and Harvard postdoc Daniel Berger.
The ultimate goal, supported by the National Institutes of Health BRAIN Initiative , is to create a comprehensive, high-resolution map of a mouse’s neural wiring, which would entail about 1,000 times the amount of data the group just produced from the 1-cubic-millimeter fragment of human cortex.
“The word ‘fragment’ is ironic,” Lichtman said. “A terabyte is, for most people, gigantic, yet a fragment of a human brain — just a minuscule, teeny-weeny little bit of human brain — is still thousands of terabytes.”

Jeff Lichtman.
Kris Snibbe/Harvard Staff Photographer
The latest map contains never-before-seen details of brain structure, including a rare but powerful set of axons connected by up to 50 synapses. The team also noted oddities in the tissue, such as a small number of axons that formed extensive whorls. Because the sample was taken from a patient with epilepsy, the researchers don’t know whether such formations are pathological or simply rare.
Lichtman’s field is connectomics, which seeks to create comprehensive catalogs of brain structure, down to individual cells. Such completed maps would unlock insights into brain function and disease, about which scientists still know very little.
Google’s state-of-the-art AI algorithms allow for reconstruction and mapping of brain tissue in three dimensions. The team has also developed a suite of publicly available tools researchers can use to examine and annotate the connectome.
“Given the enormous investment put into this project, it was important to present the results in a way that anybody else can now go and benefit from them,” said Google collaborator Viren Jain.
Next the team will tackle the mouse hippocampal formation, which is important to neuroscience for its role in memory and neurological disease.
Share this article
You might like.
Amazon immersion fosters partnerships, offers students, researchers hard look at threats to economic security, environment of rainforest as Earth warms

Senior integrative biology concentrator spots 121 species during research, teaching intensive in Amazon

Climate activist urges people to counter a culture run on fear and fossil fuel
Drug-free nasal spray blocks, neutralizes viruses, bacteria
In preclinical studies, spray offered nearly 100% protection from respiratory infections by COVID-19, influenza, viruses, and pneumonia-causing bacteria
Falls put older adults at increased risk of Alzheimer’s
Researchers found dementia more frequently diagnosed within one year of a fall, compared to other types of injuries
A blueprint for better conversations
After months of listening and learning, open inquiry co-chairs detail working group’s recommendations
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- What is brain health...
What is brain health and why is it important?
Read our brain health collection.
- Related content
- Peer review
- Yongjun Wang , professor 1 2 ,
- Yuesong Pan , associate professor 1 2 ,
- Hao Li , professor 1 2
- 1 Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2 China National Clinical Research Center for Neurological Diseases, Beijing, China
- Correspondence to: Y Wang yongjunwang{at}ncrcnd.org.cn
Yongjun Wang and colleagues discuss the definition of brain health and the opportunities and challenges of future research
The human brain is the command centre for the nervous system and enables thoughts, memory, movement, and emotions by a complex function that is the highest product of biological evolution. Maintaining a healthy brain during one’s life is the uppermost goal in pursuing health and longevity. As the population ages, the burden of neurological disorders and challenges for the preservation of brain health increase. It is therefore vital to understand what brain health is and why it is important. This article is the first in a series that aims to define brain health, analyse the effect of major neurological disorders on brain health, and discuss how these disorders might be treated and prevented.
Definition of brain health
Currently, there is no universally recognised definition of brain health. Most existing definitions have only a general description of normal brain function or emphasise one or two dimensions of brain health. The US Centers for Disease Control and Prevention defined brain health as an ability to perform all the mental processes of cognition, including the ability to learn and judge, use language, and remember. 1 The American Heart Association/American Stroke Association (AHA/ASA) presidential advisory defined optimal brain health as “average performance levels among all people at that age who are free of known brain or other organ system diseases in terms of decline from function levels, or as adequacy to perform all activities that the individual wishes to undertake.” 2
The brain is a complex organ and has at least three levels of functions that affect all aspects of our daily lives: interpretation of senses and control of movement; maintenance of cognitive, mental, and emotional processes; and maintenance of normal behaviour and social cognition. Brain health may therefore be defined as the preservation of optimal brain integrity and mental and cognitive function at a given age in the absence of overt brain diseases that affect normal brain function.
Effect of major neurological disorders on brain health
Several neurological disorders may disrupt brain function and affect humans’ health. Medically, neurological disorders that cause brain dysfunction can be classified into three groups:
Brain diseases with overt damage to brain structures, such as cerebrovascular diseases, traumatic brain injury, brain tumours, meningitis, and communication and sensory disorders
Functional brain disorders with detectable destruction of brain connections or networks, such as neurodegenerative diseases (eg, Parkinson’s disease, Alzheimer’s disease, and other dementias) and mental disorders (eg, schizophrenia, depression, bipolar disorder, alcoholism, and drug abuse)
Other brain disorders without detectable structural or functional impairment, such as migraine and sleep disorders.
These neurological disorders may have different or common effects on brain health and function. For instance, Alzheimer’s disease is the main type of dementia, with a decline in different domains of cognitive function. Mood disorders may cause dysfunction in execution, reward processing, and emotional regulations. In addition to physical disability, aphasia, gait and balance problems, and cerebrovascular diseases may lead to cognitive impairment and dementia, which are neglected by both patients and physicians.
Ageing and burden of neurological disorders
Human ageing is mainly reflected in the aspects of brain ageing and degradation of brain function. The number of people aged 60 years and over worldwide was around 900 million in 2015 and is expected to grow to two billion by 2050. 3 With the increases in population ageing and growth, the burden of neurological disorders and challenges to the preservation of brain health steeply increase. People with neurological disorders will have physical disability, cognitive or mental disorders, and social dysfunction and be a large economic burden.
Globally, neurological disorders were the leading cause of disability adjusted life years (276 million) and the second leading cause of death (9 million) in 2016, according to the Global Burden of Diseases study. 4 Stroke, migraine, Alzheimer’s disease and other dementias, and meningitis are the largest contributors to neurological disability adjusted life years. 4 About one in four adults will have a stroke in their lifetime, from the age of 25 years onwards. 5 Roughly 50 million people worldwide were living with dementia in 2018, and the number will more than triple to 152 million by 2050. 6 In the following decades, governments will face increasing demand for treatment, rehabilitation, and support services for neurological disorders.
Opportunities and challenges of future research on brain health
Opportunities and challenges exist in the assessment of brain health, the mechanism of brain function and dysfunction, and approaches to promote brain health ( box 1 ).
Lack of metrics or tools to comprehensively assess or quantify brain health
Little knowledge about the mechanisms of brain function and dysfunction
Few effective approaches to prevent and treat brain dysfunction in some major neurological disorders, such as dementia
Need to precisely preserve brain functions for people with neurosurgical diseases
Defining and promoting optimal brain health require the scientific evaluation of brain health. However, it is difficult to comprehensively evaluate or quantify brain health through one metric owing to the multidimensional aspects of brain health. Many structured or semistructured questionnaires have been developed to test brain health by self-assessments or close family member assessments of daily function or abilities. In recent decades new structural and functional neuroimaging techniques have been applied to evaluate brain network integrity and functional connectivity. 7 However, these subjective or objective measures have both strengths and weaknesses. For instance, scales such as the mini-mental state examination and Montreal cognitive assessment are simple and easy to implement but are used only as global screening tools for cognitive impairment; tests such as the digit span, Rey-Osterrieth complex figure test, trail making A and B, Stroop task, verbal fluency test, Boston naming test, and clock drawing test are used mainly to assess one or two specific domains of memory, language, visuospatial, attention, and executive function; and neuroimaging techniques, although non-invasive and objective, still have disadvantages of test contraindications, insufficient temporal or spatial resolution, motion artefact, and high false discovery rates, which limit their clinical transformation.
Another difficulty in measuring brain health is that age, culture, ethnicity, and geography specific variations exist in the perception of optimal brain health. Patient centred assessment of brain function, such as self-perception of cognitive function and quality of life, should also be considered when measuring brain health. 8 Universal acceptable, age appropriate, multidimensional, multidisciplinary, and sensitive metrics or tools are required to comprehensively measure and monitor brain function and brain health.
To promote optimal brain health, we need a better understanding of the mechanisms of brain function and dysfunction. Unfortunately, little is known about the working mechanism of the brain. Although we have made considerable developments in neuroscience in recent decades, we still cannot totally decipher the relations between spatiotemporal patterns of activity across the interconnected networks of neurons and thoughts or the cognitive and mental state of a person. 9 Recent progress in brain simulation and artificial intelligence provides a vital tool to understand biological brains, and vice versa. 10 11 The development of brain inspired computation, brain simulation, and intelligent machines was emphasised in the European Union and China Brain Project. 9 12
Meanwhile, the mechanisms behind the brain dysfunction in some neurological disorders are still not well understood, especially for mental and neurodegenerative disorders. Further investigation of the mechanisms of brain diseases may indicate approaches to treatment and improve brain function. Brain imaging based cognitive neuroscience may unravel the underlying brain mechanism of cognitive dysfunction and provide an avenue to develop a biological framework for precision biomarkers of mood disorders. 13
Most common neurological diseases, such as cerebrovascular diseases and Alzheimer’s disease, have complex aetiopathologies, typically involving spatial-temporal interactions of genetic and environmental factors. However, a single genetic factor could account for the disease progression of monogenic neurological disorders. These diseases could be more readily investigated by simplified cross species modelling, leading to better understanding of their mechanisms and greater efficiency in testing innovative therapies. Such research may provide a window to promote the investigation of common neurological disorders and general brain health, as discussed by Chen and colleagues elsewhere in this series. 14
Few effective approaches are available to prevent and treat brain dysfunction in some major neurological disorders, such as dementia. Neurons are not renewable, and brain dysfunction is always irreversible. Recent trials targeting amyloid clearance and the selective inhibition of tau protein aggregation failed to improve cognition or modify disease progression in patients with mild Alzheimer’s disease. 15 16 More attention has focused on other potential therapeutic targets, such as vascular dysfunction, inflammation, and the gut microbiome, as discussed by Shi and colleagues. 17 In particular, recent studies showed that the early impairment of cognition was induced by the disruption of neurovascular unit integrity, which may cause hypoperfusion and the breakdown of the blood-brain barrier and subsequent impairment in the clearance of proteins in the brain. 18 19 Physical activity, mental exercise, a healthy diet and nutrition, social interaction, ample sleep and relaxation, and control of vascular risk factors are considered six pillars of brain health. The AHA/ASA presidential advisory recommended the AHA’s Life’s Simple 7 (non-smoking, physical activity, healthy diet, appropriate body mass index, blood pressure, total cholesterol, and blood glucose) to maintain optimal brain health. 2 Pan and colleagues discuss how this may indicate a new dawn of preventing some cognitive impairment and brain dysfunction by preventing vascular risk factors or cerebrovascular diseases. 20
For other neurological disorders with potential therapeutic approaches, the main aim is to preserve brain function. Impaired brain function due to anatomical structural damage is underestimated in patients with neurosurgical diseases such as brain tumours, trauma, and epilepsy. In recent years, treatment targets for neurosurgical diseases have changed from focusing on survival or life expectancy to balancing brain structures and functions. Precise preservation of brain function requires an understanding of the exquisite relation between brain structure and function and advanced technologies to visualise brain structure-function relations. 21
Another example of the predicament associated with protection of brain function is uncertainty in the treatment response in epilepsy management. Current standard care for epilepsy relies on a trial and error approach of sequential regimens of antiseizure medications. The time delay due to this treatment approach means that such treatments may be less effective and irreversible damage may occur. Chen and colleagues 22 describe how recent advances in personalised epilepsy management based on artificial intelligence, genomics, and patient derived stem cells are bringing some hope to overcome this predicament in epilepsy management and promise a more effective strategy. 23 24
Brain health is the maintenance of multidimensional aspects of brain function. However, several neurological disorders may affect brain health in one or more aspects of brain function. Deciphering and promoting the function and health of the brain, the most mysterious organ in the human body, will have a dramatic impact on science, medicine, and society. 25 In the past seven years, a number of large scale brain health initiatives have been launched in several countries to promote the development of neuroscience, brain simulation, and brain protection. 9 However, further challenges are raised by the different key research directions of brain projects in different countries. In the face of these challenges, Liu and colleagues argue that collaboration on brain health research is urgently needed. 26 As the other articles in this series describe, coordinated research has enormous potential to improve the prognosis of brain disorders.
Key messages
Brain health is the preservation of optimal brain integrity and mental and cognitive function and the absence of overt neurological disorders
Human ageing increases the burden of brain dysfunction and neurological diseases and the demands for medical resources
Further studies are required to assess brain health, understand the mechanism of brain function and dysfunction, and explore effective approaches to promote brain health.
Contributors and sources: YW proposed the idea for this series on brain health. YW and YP drafted the first manuscript. All the authors critically reviewed and revised the manuscript. YP and HL expertise is in the area of clinical research methods and clinical research on stroke. YW is an expert in clinical research on stroke and neurological diseases. YW is the guarantor.
Competing interests We have read and understood BMJ policy on declaration of interests and declare that the study was supported by grants from the National Science and Technology Major Project (2017ZX09304018), National Key R&D Program of China (2018YFC1312903, 2017YFC1310902, 2018YFC1311700, and 2018YFC1311706), National Natural Science Foundation of China (81971091), Beijing Hospitals Authority Youth Programme (QML20190501), and Beijing Municipal Science and Technology Commission (D171100003017002).
Provenance and peer review: Commissioned; externally peer reviewed.
This article is part of a series launched at the Chinese Stroke Association annual conference on 10 October 2020, Beijing, China. Open access fees were funded by the National Science and Technology Major Project. The BMJ peer reviewed, edited, and made the decision to publish these articles.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- ↵ Centers for Disease Control and Prevention. Healthy aging. What is a healthy brain? New research explores perceptions of cognitive health among diverse older adults. https://www.cdc.gov/aging/pdf/perceptions_of_cog_hlth_factsheet.pdf
- Gorelick PB ,
- Iadecola C ,
- American Heart Association/American Stroke Association
- ↵ WHO Global Health Ethics team. Ageing. https://www.who.int/ethics/topics/ageing/en/ . 2019
- GBD 2016 Neurology Collaborators.
- Feigin VL ,
- GBD 2016 Lifetime Risk of Stroke Collaborators
- ↵ Alzheimer’s Disease International. World Alzheimer report 2018. The state of the art of dementia research: new frontiers. https://www.alz.co.uk/research/world-report-2018
- Gordon MF ,
- Lenderking WR ,
- Patient-Reported Outcome Consortium’s Cognition Working Group
- Ullman TD ,
- Tenenbaum JB ,
- Gershman SJ
- Hassabis D ,
- Kumaran D ,
- Summerfield C ,
- Botvinick M
- Capitão L ,
- Satterthwaite TD ,
- Thomas RG ,
- Alzheimer’s Disease Cooperative Study Steering Committee ,
- Solanezumab Study Group
- Gauthier S ,
- Feldman HH ,
- Schneider LS ,
- Sabbagh MN ,
- Henstridge CM ,
- Spires-Jones TL
- Sweeney MD ,
- Montagne A ,
- Zlokovic BV
- Wardlaw JM ,
- Couldwell WT ,
- Antonic-Baker A ,
- Kuhlmann L ,
- Lehnertz K ,
- Richardson MP ,
- Schelter B ,
- Epi4K Consortium
- Schwamm LH ,
- Koroshetz WJ
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine magazine explores the brain and nervous system
The new issue of Stanford Medicine magazine features articles about developments in neuroscience and treatments for conditions affecting the brain and nervous system.
October 14, 2021 - By Rosanne Spector
The new issue of Stanford Medicine magazine focuses on how researchers are unlocking secrets of the brain. Illustration by Craig Cutler
The brain has long been a black box and, until recently, we were in the dark about anything that might have gone wrong under its lid and what to do about it. That’s changing.
A themed section of the new issue of Stanford Medicine magazine, “The most mysterious organ: Unlocking the secrets of the brain,” provides new insights into neurological conditions ranging from Alzheimer’s disease to stroke and conveys clinicians’ optimism about the relatively recent understanding that the brain is surprisingly adaptable. As Lloyd Minor, MD, dean of the Stanford University School of Medicine, wrote in his letter to readers :
“One of our most fascinating discoveries is that the brain isn’t as fixed and fragile as we once believed. The organ we thought was set in its ways by our late 20s is much more active — and resilient — for our entire lives.”
Advances in brain imaging and a more accurate understanding of the brain’s workings are enabling researchers and health care practitioners to develop new treatments. Some of these are available only at Stanford Medicine through clinical trials, while others have been adopted around the world.
The issue includes:
• A roundup of research and treatments aimed at addressing diseases of the brain and nervous system. These advances are enabling the paralyzed to move and the blind to see . They’re also suggesting strategies for preventing the loss of cognitive abilities .
• An article about brain trauma data from the U.S. Department of Veterans Affairs revealing that women have a more difficult recovery from severe brain trauma than men do. The article is accompanied by a video featuring neurosurgery faculty members Odette Harris, MD, and Maheen Adamason, PhD, and a veteran talking about her experience of severe brain trauma.
• A story of the decadeslong quest to save more stroke patients from a life of disability. It was a tough sell to other neurosurgeons, but research by Gregory Albers, MD, and a team of researchers eventually succeeded in extending the window for effective treatment from just a few hours to a full day.
• A recounting of a high-stakes, innovative surgery to save a toddler’s life by removing a brain tumor through his nose .
• A Q&A with renowned flutist Eugenia Zukerman in which she reflects on the healing power of writing during the early stages of Alzheimer’s disease, her first book of poetry and the hope her poetry offers other patients.
• An article about an experimental treatment for Parkinson’s disease that seems like magic: a vibrating glove that reduces symptoms for patients who wear it a few hours a day.
• An essay adapted from a new book by psychiatry professor Karl Deisseroth , MD, PhD, Projections : A Story of Human Emotions , that describes an encounter with a young man who couldn’t cry and the neurological mechanisms behind shedding tears of joy and sorrow.
• A story about the efforts of neurosurgery chair Michael Lim, MD, to apply the successes of immunotherapy cancer treatments to some of the most pernicious tumors — those that originate in the brain.
• An article about an unusual surgery for a rare disease: Hope Kim needed a second bypass surgery to treat the brain disorder moyamoya , but no blood vessels in the scalp were right for the job. What to do? Professor of neurosurgery Gary Steinberg, MD, PhD, piped a blood vessel from her abdomen up to her brain.
This issue also includes a profile of associate professor of bioengineering Drew Endy, PhD, who believes that solving civilization’s most vexing challenges depends on harnessing “bioengineering to flourish in partnership with nature,” and excerpts from The Puzzle Solver , by Stanford Medicine science writer Tracie White with professor of genetics and of biochemistry Ron Davis, PhD. The book describes Davis’ desperate attempt to find a cure for severe chronic fatigue syndrome, also known as myalgic encephalomyelitis, which has left his son bedridden by pain, fatigue and other disabling symptoms.
Stanford Medicine magazine is available online at stanmed.stanford.edu as well as in print. Print copies of the new issue are being sent to subscribers. Others can request a copy by sending an email to [email protected] .

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
The majestic cell
How the smallest units of life determine our health


What neuroscience tells us about the teenage brain
New research now turns an old assumption on its head, as psychologists seek to optimize social contexts and environments for developing minds
Vol. 53 No. 5 Print version: page 66
- Cognition and the Brain

For years, the teenage brain was seen by researchers, policymakers, and the public as more of a burden than an asset. Adolescents were risk machines who lacked the decision-making powers of a fully developed prefrontal cortex—and liable to harm themselves and others as a result. That narrative is beginning to change.
There is growing recognition that what was previously seen as immaturity is actually a cognitive, behavioral, and neurological flexibility that allows teens to explore and adapt to their shifting inner and outer worlds.
Developmental cognitive neuroscientists are at the frontier of this new outlook, using updated methodology, larger and more diverse samples, and experimental tasks with real-world relevance to answer questions about adolescents in the context of society. They’re also supporting developmentally informed policy and practice on everything from mental health care to juvenile justice. “The adolescent brain was long portrayed as broken, immature, or contributing to problematic behaviors,” said Eva Telzer, PhD, an associate professor of psychology and director of the Developmental Social Neuroscience Lab at the University of North Carolina, Chapel Hill. “But in the last five years, there’s been a huge shift toward seeing the developing brain as malleable, flexible, and promoting many positive aspects of development in adolescence.”
Heightened sensitivity to rewards, for example, which is partly driven by increased activity in a part of the brain called the ventral striatum, has been implicated in behaviors such as substance use and unprotected sex among teens. But research now shows that in different settings, that same neural circuitry can also promote positive peer influence and behaviors, Telzer said, such as wearing a seat belt or joining a peaceful protest.
As the field of developmental neuroscience matures, so too do the questions researchers ask. Studies are increasingly considering the influence not just of peers but also of parents. Researchers are also looking closely at how social media use may affect young brains, as concerns mount about teens’ online activity. As a result, research on the teenage brain is finally starting to catch up with studies of other age groups, complete with the level of detail it deserves.
“The shift from childhood to adulthood is not a linear one. Adolescence is a time of wonderfully dynamic change in the brain,” said BJ Casey, PhD, a professor of psychology who directs the Fundamentals of the Adolescent Brain Lab at Yale University. “Too often, we’ve superimposed an adult model onto a developing brain, but now we’re starting to see more nuanced findings.”
Embracing new approaches
Adolescence—spanning from puberty until the mid-20s—describes the transitional period between childhood and adulthood, according to the National Academies of Sciences, Engineering, and Medicine . During this period, the brain grows and changes in a number of ways. Gray matter in the cerebral cortex tends to thin, while white matter that connects various regions of the brain generally increases in volume. Functional connections between regions, which researchers measure with brain scans that track oxygen usage in blood, also undergo widespread changes during adolescence.
Beyond that, things get a little more complicated—and recent replication efforts indicate that some findings considered fundamental to the field may not hold up in larger samples. For example, early research suggested that brain volume increases peaked earlier in adolescent girls (Lenroot, R. K., & Giedd, J. N., Brain and Cognition , Vol. 72, No. 1, 2010 ), but more recent studies of large, international samples have shown it’s not that simple. Instead, boys’ brains tend to change at similar rates regardless of variability in other brain metrics, while changes in girls’ brains can be predicted based on certain measurements, such as the thickness of the cortex (Mills, K. L., et al., NeuroImage , Vol. 242, 2021 ).
“This kind of finding is emblematic of a bigger shift in the field as to how we’re approaching our science, what techniques we use, and what information we consider valuable,” said Jennifer Pfeifer, PhD, a professor of psychology and director of the University of Oregon’s Developmental Social Neuroscience Lab . “It’s become clear that if we want to understand developmental processes within individuals, we need to use some different tactics.”
More sophisticated methodology is a big part of that shift, she added. Instead of merely comparing brain structure or activity between two age groups (12- and 18-year-olds, for instance), researchers are increasingly relying on a variety of experimental approaches that follow the same youth over time.
“Many people may not realize that our early insights about adolescent brain development were based on cross-sectional approaches, which can sometimes lead to the wrong conclusions,” said Pfeifer, who also codirects the National Scientific Council on Adolescence .
Now, researchers use other techniques, such as accelerated longitudinal designs, where participants are sampled a handful of times at a range of ages (starting at ages 12 to 15, for instance, and then annually for three years), which can paint a more comprehensive picture of neurodevelopment.
Large collaborative consortia, in particular the Adolescent Brain Cognitive Development (ABCD) Study, a decade-long effort that follows a nationally representative sample of nearly 12,000 teens, are also providing richer data that can power more rigorous studies of the developing brain. The ABCD Study shares its brain scans measuring neurological development, clinical tests of mental and physical health, and behavioral data on substance use, academic achievement, and more with researchers around the world; of about 250 papers published using the survey’s data so far, half were from investigators outside the consortium. Fittingly, one of the study’s early insights is that very large samples—with thousands of individual brain scans—are needed to detect reproducible differences between individuals at the whole-brain level (Marek, S., et al., Nature , Vol. 603, 2022 ).
“On the other hand, there is still a need for innovative, smaller-scale studies,” said Eveline Crone, PhD, a professor of neurocognitive developmental psychology and director of the Society, Youth, and Neuroscience Connected (SYNC) Lab at Erasmus University Rotterdam in the Netherlands. “If we only ran the big consortia, we would miss out on a lot of novelty in terms of our methods and research questions.”
Some questions—for instance, how adolescents’ brains respond, on average, to winning money for themselves, a family member, or a stranger—can be examined effectively in much smaller samples, pointing to the importance of a balance between large and small efforts ( Developmental Cognitive Neuroscience , Vol. 51, 2021 ). (Crone and her colleagues who conducted this research have found that teen brains show activation in the nucleus accumbens, part of the brain’s reward system, when achieving gains for themselves or their parents but not for strangers. Those findings point to the development of ingroup-outgroup distinctions during adolescence.) Like others in the field, Crone employs a mixed-methods approach, combining brain imaging with behavioral measures, youth panels, and large-scale surveys to contextualize development alongside behavior, relationships, and society.
Another major advance is the creation and use of “ecologically valid” experimental tasks, or those that more accurately mimic teens’ experiences outside the lab.
“If we really want to understand how the adolescent brain works, we need to use stimuli—things like social media and video games—that they actually care about,” said Jennifer Silk, PhD, a professor of psychology at the University of Pittsburgh who runs the Families, Emotions, Neuroscience, and Development Lab .
Silk and her colleagues have developed one such activity, called the Chatroom Interact Task, which simulates acceptance and rejection from peers. Teenage girls participating in the task are either “chosen” or “rejected” by other girls their age while undergoing an fMRI scan, which maps brain activity by measuring changes in blood flow and oxygen levels. Other tasks monitor teens while they use platforms similar to Instagram and Facebook, including how their brains respond to receiving “likes.”
Researchers are even collecting data that may redefine the meaning of “adolescence,” with an eye on the juvenile justice system.
“We’re expanding the age ranges we’re looking at because the field is recognizing that significant neurocognitive changes continue into the 20s,” said Casey. “Those changes have consequences with regard to decision-making,” and research in this area may ultimately inform more scientifically aligned approaches to reward, punishment, justice reform, and other areas.
Optimizing mental health
Teens are famous for their heightened emotional sensitivity, especially in social interactions. Researchers are starting to pin down brain circuitry linked to that sensitivity—and differentiate between cases where it’s an asset that helps teens reach emotional maturity versus a risk factor that may predict mental health problems (Casey, B. J., et al., Neuroscience Letters , Vol. 693, 2019 ).
Research by Silk, Telzer, Casey, and others has identified several areas of the brain that underlie emotional responses in teens, including the subgenual cingulate cortex, anterior insula, and amygdala. For example, teens who had more activity in those regions during the rejection phase of Silk’s Chatroom Interact Task, compared with the acceptance phase, were more likely to experience depression and suicidality down the line ( Journal of Clinical Child & Adolescent Psychology , online first publication, 2022 ; Child Psychiatry & Human Development , Vol. 51, 2020 ). “There seems to be some sensitivity to rejection in this brain network that’s related to the development of internalizing disorders,” Silk said.
Because mental health problems increase sharply during adolescence—affecting an estimated 1 in 4 teens—there’s an urgent need to determine who is at risk and what treatments may be most effective (Silva, S. A., et al., PLOS ONE , Vol. 15, No. 4, 2020 ).
In animal models, stressful experiences during adolescence appear to alter the development of emotion-focused regions such as the amygdala and hippocampus, as well as the prefrontal cortex (Eiland, L., & Romeo, R. D., Neuroscience , Vol. 249, 2013 ). Early findings from the ABCD Study have found different patterns of activation in the amygdala, anterior cingulate cortex, and other reward-associated brain regions among preteens with disruptive behavior disorders, as well as brain differences that may underlie attention-deficit/hyperactivity disorder (ADHD) (Hawes, S. W., et al., The American Journal of Psychiatry , Vol. 178, No. 4, 2021 ; Bernanke, J., et al., The Lancet Psychiatry , Vol. 9, No. 3, 2022 ).
Rather than searching for a drug or mechanism that can address the entirety of depression, anxiety, or ADHD, researchers are increasingly studying specific symptoms—anhedonia or inattention, for instance—as well as subtypes of various disorders and seeking solutions for each.
“We are now looking at specific behaviors for which we can identify a neural circuit, mechanisms, and sometimes even genes,” said Pradeep Bhide, PhD, a professor of developmental neuroscience and director of the Center for Brain Repair at the Florida State University College of Medicine. “That is a newer, better, and likely more successful approach to treating complex human psychiatric and developmental disorders.”
For example, adolescents tend to benefit less from fear extinction efforts than adults (Pattwell, S. S., et al., PNAS , Vol. 109, No. 40, 2012 ). According to Casey, this suggests that they may respond poorly to exposure therapy, a key component of cognitive behavioral therapy (CBT) for anxiety, which recruits the prefrontal cortex to reprogram fear memories. It may therefore be possible to optimize CBT to work better for adolescents by using strategies that bypass the prefrontal cortex, instead working to alter memories using other circuitry, including emotion- and memory-focused regions such as the hippocampus and amygdala ( Scientific Reports , Vol. 5, No. 8863, 2015 ; Nature Communications , Vol. 7, No. 11475, 2016 ). This process is often referred to as “memory reconsolidation” or “reconsolidation update.”
“Thus, there appear to be developmental windows in which we can optimize treatments in specific ways,” Casey said.
Parents and peers
When it comes to teens’ relationships, both the scientific community and the lay public have long embraced the assumption that adolescence triggers a shift away from parents and toward peers, particularly when it comes to risk-taking.
New findings are challenging that assumption, which was pervasive but difficult to test directly, Pfeifer said (Nelson, E. E., et al., Psychological Medicine , Vol. 35, No. 2, 2005 ). Early data from Project NeuroTeen, Telzer’s 5-year longitudinal study of how parent and peer relationships influence adolescent decision-making and development, show that teens shift their behavior to align with the risky choices of parents more than the risky choices of peers. This shift is supported by increased activation in regions of the brain related to reward, including the ventral striatum and ventromedial prefrontal cortex ( Journal of Research on Adolescence , Vol. 31, No. 1, 2021 ).
Silk, Amanda Morris, PhD, of Oklahoma State University, and their colleagues have started to document the synchrony between teens and their parents in real time, using a new simultaneous scanning technique to measure how one brain responds to another during an interaction. They have found that adolescent brain activity tends to mirror parent brain activity, especially in emotion-processing regions such as the amygdala and anterior insula ( Child Development , Vol. 92, No. 6, 2021 ).
“I think a lot of parents believe that it’s too late, that by adolescence, peers have all the power,” Silk said. “But this research is showing that parents shouldn’t give up, that they still do have the power to help their adolescents learn how to process and regulate their emotions.”
Pfeifer’s lab also recently explored the claim that changes in the brain during adolescence make teens more sensitive to social information related to acceptance by peers, but their findings did not clearly support that idea. Instead, they found that activity in regions such as the ventromedial prefrontal cortex—a brain area related to evaluation of the self—tended to peak during mid-adolescence, especially for information related to status (Cosme, D., et al., Developmental Cognitive Neuroscience , Vol. 54, 2022 ). These findings may suggest that “identity is an important source of value to adolescents, and this could be leveraged to promote healthy decision-making,” she said ( Child Development Perspectives , Vol. 12, No. 3, 2018 ).
Peer interactions are still important, of course, and they’re increasingly happening online. Parents, researchers, and policymakers have plenty of unanswered questions about how social media use may affect the developing brain. For example, do certain neural profiles among teens predict riskier online behavior, such as the tendency to compare one’s appearance and social status to others?
Silk and Cecile Ladouceur, PhD, of the University of Pittsburgh, have launched new research to bring more nuance to that conversation. They are collecting information about social media use from teens’ phones, along with fMRI data on their neurological responses to acceptance and rejection, for instance during the Chatroom Interact Task. Telzer has also launched a new effort, with Mitch Prinstein, PhD, APA’s chief science officer, to study whether brain development in regions responsible for reward, emotion, and cognitive control relates to how frequently teens check their social media apps.
“Undoubtedly there’s a link between teens’ social experiences online and the way their brains respond to the environment, but it’s something we’re slowly working to unpack,” Telzer said.
Challenging assumptions about teens
The malleability of the adolescent brain may make it vulnerable at times, but teen brains are also highly capable of prosocial growth under the right circumstances, Pfeifer said. Teens’ biological need for social connection, combined with their heightened sensitivity to rewards, likely underlies teen-led activism, for instance on climate change, racial justice, and gun control.
Research by Crone and others shows that the ventral striatum is linked to prosocial behavior, responding to rewards not just for oneself but also for others ( Nature Communications , Vol. 12, No. 313, 2021 ). Among teens serving time in youth detention centers, both the ability to spontaneously take the perspective of others and activity in the temporoparietal junction—an associated region of the brain—differed significantly from a control group. The temporoparietal junction is more malleable by environmental influence than other social brain regions, according to studies by Crone among twin populations. This suggests that interventions in perspective-taking, which target this area, may be helpful for justice-involved teens ( Social Cognitive and Affective Neuroscience , Vol. 9, No. 12, 2014 ).
Based on ongoing research by Casey and others about the trajectory of development in regions related to cognitive control, including the prefrontal cortex, APA has launched a task force to review new findings that may inform extending Roper v. Simmons , a Supreme Court decision that abolished the use of the death penalty for those under 18, to cover individuals into their early 20s.
Looking forward, researchers in the field emphasize the importance of continuing to challenge assumptions about adolescence—around risk-taking, emotionality, and more—to ensure that the science remains robust and can ultimately support interventions for healthy development.
“We’re not going to change adolescents’ brains, nor should we want to,” Telzer said. “What we can do is optimize what we know to create social contexts and environments that provide the most enriching experiences for them.”
Further reading
Why young brains are especially vulnerable to social media Abrams, Z., APA, 2022
A deep dive into adolescent development Weir, K., Monitor on Psychology , June 2019
Justice for teens Stringer, H., Monitor on Psychology , October 2017
Teens aren’t just risk machines—there’s a method to their madness Flannery, J., et al., The Conversation , February 6, 2018
Recommended Reading

Contact APA
- Psychology topic: Teens
You may also like
- Advanced search

Advanced Search
How Behavior Shapes the Brain and the Brain Shapes Behavior: Insights from Memory Development
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Figures & Data
- Info & Metrics
Source memory improves substantially during childhood. This improvement is thought to be closely related to hippocampal maturation. As previous studies have mainly used cross-sectional designs to assess relations between source memory and hippocampal function, it remains unknown whether changes in the brain precede improvements in memory or vice versa. To address this gap, the current study used an accelerated longitudinal design ( n = 200, 100 males) to follow 4- and 6-year-old human children for 3 years. We traced developmental changes in source memory and intrinsic hippocampal functional connectivity and assessed differences between the 4- and 6-year-old cohorts in the predictive relations between source memory changes and intrinsic hippocampal functional connectivity in the absence of a demanding task. Consistent with previous studies, there were age-related increases in source memory and intrinsic functional connectivity between the hippocampus and cortical regions known to be involved during memory encoding. Novel findings showed that changes in memory ability early in life predicted later connectivity between the hippocampus and cortical regions and that intrinsic hippocampal functional connectivity predicted later changes in source memory. These findings suggest that behavioral experience and brain development are interactive, bidirectional processes, such that experience shapes future changes in the brain and the brain shapes future changes in behavior. Results also suggest that both timing and location matter, as the observed effects depended on both children's age and the specific brain ROIs. Together, these findings add critical insight into the interactive relations between cognitive processes and their underlying neurologic bases during development.
SIGNIFICANCE STATEMENT Cross-sectional studies have shown that the ability to remember the contextual details of previous experiences (i.e., source memory) is related to hippocampal development in childhood. It is unknown whether hippocampal functional changes precede improvements in memory or vice versa. By using an accelerated longitudinal design, we found that early source memory changes predicted later intrinsic hippocampal functional connectivity and that this connectivity predicted later source memory changes. These findings suggest that behavioral experience and brain development are interactive, bidirectional processes, such that experience shapes future changes in the brain and the brain shapes future behavioral changes. Moreover, these interactions varied as a function of children's age and brain region, highlighting the importance of a developmental perspective when investigating brain-behavior interactions.
- accelerated longitudinal design
- brain development
- episodic memory
- hippocampal functional connectivity
- memory development
- source memory
- Introduction
Source memory improves substantially during childhood (e.g., Riggins, 2014 ). Specifically, with age, children become better at reporting and retaining contextual details of life experiences ( Bauer, 2007 ). This development is closely related to hippocampal maturation, as evidenced by age- and memory-related differences in hippocampal structure and function across development (see Ghetti and Bunge, 2012 for review; Sastre et al., 2016 ; Tang et al., 2018 ; Riggins et al., 2020 ). Previous studies have mainly used cross-sectional designs to assess relations between brain and memory development, which do not allow for investigating true developmental changes and may be influenced by confounding factors, such as cohort effects. Therefore, it remains unknown whether changes in the brain precede improvements in memory or vice versa. Additionally, previous studies have focused on either young children or school-aged children, which make comparisons of different developmental periods difficult. To address these gaps, the current study used an accelerated longitudinal design to follow 4- and 6-year-old children for 3 years. This design allowed us to explore developmental changes in intrinsic hippocampal functional connectivity (iHFC) and to assess differences between the 4- and 6-year-old cohorts in the predictive relations between source memory and functional connectivity (see Fig. 1 ).
- Download figure
- Open in new tab
- Download powerpoint
Overview of analyses examining concurrent and predictive relations between source memory and iHFC across development.
In adults, intrinsic functional connectivity is thought to reflect the brain's functional architecture, which emerges as a result of task-elicited coactivation between brain regions ( Fox and Raichle, 2007 ). Children's intrinsic functional connectivity patterns are likely constructed in a similar manner; however, the long-term molding hypothesis has proposed that these connectivity patterns are shaped over time as a result of both maturation and experience ( Gabard-Durnam et al., 2016 ). For example, by following 4- to 18-year-olds over 2 years, prospective analyses indicated that task-elicited amygdala functional connectivity predicted resting-state functional connectivity 2 years later, but not concurrently ( Gabard-Durnam et al., 2016 ). These findings suggest associations between task-based brain activation and intrinsic functional connectivity in both children and adults; however, such associations may differ across development.
Empirical data also support bidirectional influences between the brain and behavior. First, previous studies support the notion that behavioral changes shape task-based and intrinsic functional connectivity (e.g., Jolles et al., 2016 ; Clark et al., 2017 ; Rosenberg-Lee et al., 2018 ). For example, in 8- to 9-year-old children, 8 weeks of math tutoring strengthened iHFC to intraparietal sulcus ( Jolles et al., 2016 ). Second, empirical studies have shown that intrinsic functional connectivity can predict gains in cognitive abilities later in development (e.g., Hoeft et al., 2011 ; Supekar et al., 2013 ; Evans et al., 2015 ). For example, Supekar et al. (2013) found that iHFC measured before math tutoring predicted performance improvements after tutoring during middle childhood.
Based on these studies, we explored whether there were bidirectional influences between source memory changes and intrinsic functional connectivity from the hippocampus to brain regions reported to support encoding contextual information ( Geng et al., 2019 ). We used an accelerated longitudinal design to assess the following: (a) age-related changes in concurrent relations between source memory and iHFC; (b) predictive relations between early source memory changes and later iHFC; and (c) predictive relations between early iHFC and later source memory changes ( Fig. 1 ).
We hypothesized that early source memory gains would predict later iHFC because the impact of experience is thought to build up over time ( Gabard-Durnam et al., 2016 ). Accumulating experiences with everyday memory activities were expected to drive developmental changes in iHFC during childhood. Additionally, because of greater plasticity early in development (e.g., Tottenham and Sheridan, 2010 ), we expected that memory changes would more robustly predict later connectivity in the younger versus older cohort.
As brain connectivity has been suggested to shape later behavior (e.g., Evans et al., 2015 ), we hypothesized that iHFC would predict gains in source memory abilities. Additionally, because hippocampal function is more mature during middle versus early childhood ( Geng et al., 2019 ), we hypothesized that connectivity at 6 years would more robustly predict future memory than connectivity at 4 years.
- Materials and Methods
Participants
Children were participants in a large study investigating memory and brain development in early childhood that used an accelerated longitudinal design ( N = 200, 100 males) ( Riggins et al., 2018 ; Geng et al., 2019 ). The first wave (W1) of the study included 4- to 8-year-old children. The 4- and 6-year-old children were invited back for two subsequent waves of testing (W2 and W3; Fig. 2 ). In total, there were three waves, and each wave included young and old cohorts (young cohort: W1 = 4 years, W2 = 5 years, W3 = 6 years; old cohort: W1 = 6 years, W2 = 7 years, W3 = 8 years). Table 1 shows the number of children who provided 3, 2, or 1 waves of data for final analyses in each cohort. The main reasons for loss of neuroimaging data were that the children moved too much, fell asleep during the scan, refused to enter the scanner, or the families failed to follow up.
- View inline
Participants included in behavioral and neuroimaging data analyses
Number of waves children participated in the study and their age at each wave of participation.
We measured children's IQ at W1 by using the vocabulary and block design subtests from either the Wechsler Intelligence Scale for Children (Ed 4) ( Wechsler, 2003 ) or the Wechsler Preschool and Primary Scale for Intelligence ( Wechsler, 2012 ). All children included in analyses had average to above-average estimated IQ. No difference was found between the young and old cohorts in scaled scores on the vocabulary (young: mean = 11.07, SD = 2.96; old: mean = 11.66, SD = 2.55; p = 0.33) or block design subtests (young: mean = 12.96, SD = 2.73; old: mean = 13.03, SD = 3.00; p = 0.92). Parents reported all participants to be healthy without any neurodevelopmental disorders, neurologic conditions, or psychiatric conditions. Additionally, parents reported 88.5% children as righthanded, 6.8% as lefthanded, 3.7% as ambidextrous, and 1% as not able to be determined.
Experimental design
During the first visit, children learned novel facts (e.g., “A group of rhinos is called a crash”) from one of two different sources, a female adult (“Abby”) and a male-voiced puppet (“Henry”) via digital videos ( Drummey and Newcombe, 2002 ; Riggins, 2014 ). Each source provided 6 facts for a total of 12 facts. Presentation of facts was blocked by source, where children first learned 6 facts from one source followed by 6 facts from the other source, and the order of blocks was randomized across participants. There were three lists of facts; each list consisted of unique facts that were similar across lists (e.g., “A group of kangaroos is called a mob” or “A group of goats is called a tribe”). These lists were randomly assigned across participants. Children were asked to pay attention to the facts as they would be tested on the facts the following week, but were not told that they would be tested on the source of the facts. Children were asked whether they knew the facts before the experiment. Known facts were excluded and were replaced with additional novel facts from the list of the same source (but this rarely occurred). Each source had 8 possible facts to account for the possibility that children would know 1 or 2 of the facts. If a child knew 3 or more facts from one source, the total number of facts the child was tested on was reduced (but this was rare, n = 4).
During the second visit, children were tested on their memory for the facts and sources from the first visit. Children were asked to answer 22 trivia questions and to tell the experimenter where they had learned the answers to those trivia questions. They were told that they had learned some of the questions the week before from either “Abby” or “Henry,” some they might have learned outside the laboratory (e.g., from a teacher or parent), and some they may not know. The children learned 6 of the 22 facts presented from “Abby,” 6 from “Henry,” 5 were facts commonly known by children (e.g., “What color is the sky?”), and 5 were facts that children typically would not know (e.g., “What is the colored part of your eye called?”). Each list of 22 facts had two random presentation orders, and these orders were counterbalanced across participants. If children were unable to recall the source for a particular question, five multiple choice options were given: parents, teacher, girl in the video, puppet in the video, or just knew/guessed.
Imaging data acquisition
Participants were scanned in a Siemens 3.0 T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions) using a 32-channel coil. Children first completed the task-free scan, followed by a T1-weighted structural scan (T1, see Riggins et al., 2018 ). In the first wave of data collection, children also completed a memory encoding task if time permitted (for results, see Geng et al., 2019 ). During the task-free scan, children were not given any overt task but were instructed to lie as still as possible with their eyes open. The duration of the scan was 7 min and 6 s. To minimize motion, Inscapes, a movie designed to reduce head motion during fMRI data collection, was played ( Vanderwal et al., 2015 ). A total of 210 whole-brain volumes were collected using a T2*-weighted gradient EPI sequence (TR 2 s, TE 25 ms, slice thickness 3.5 mm, voxel size 3.0 mm × 3.0 mm × 3.5 mm, voxel matrix 64 × 64, flip angle 70°, FOV 192 mm, 36 slices). Structural images were acquired during a 4 min 26 s scan with a T1-weighted MPRAGE (TR 1.9 s; TE 2.32 ms; slice thickness 0.9 mm with no gap; voxel size 0.9 × 0.9 × 0.9 mm; voxel matrix 256 × 256 mm; flip angle 9°; FOV 230 × 230 mm).
During the task-free scan, participant head motion was monitored in real-time. If a participant exhibited excessive head motion (>2 mm in any direction) during the first half of any run, the scan was restarted and the participant was reminded to stay as still as possible.
Imaging data preprocessing
In the analyses, all 210 collected resting-state fMRI images were included, as the first four volumes were discarded before data collection because of the instability of the initial MR signal and participant adaptation. Preprocessing included the following steps. First, slice time correction, head motion correction, and smoothing were performed using DPABI 1.3 ( Yan et al., 2016 ). An independent component analysis was then run on smoothed data using MELODIC, an FSL toolbox, to remove artifact-related components ( Geng et al., 2019 ). After removing all artifact-related components, brain extraction, normalization, and filtering were conducted. Following the procedure suggested by Tillman et al. (2018), brain extraction on T1-weighted image was conducted separately in six toolboxes: the Advanced Normalization Tools, AFNI, FSL, BSE, ROBEX, and SPM8 to ensure high-quality data. The voxels extracted by at least four toolboxes were included in the brain mask. Advanced Normalization Tools was used to perform coregistration and normalization. Statistical analyses were conducted in AFNI ( Cox, 1996 ). Temporal bandpass filtering (0.01-0.1 Hz) and spatial smoothing with a 5 mm FWHM Gaussian kernel were performed in AFNI on normalized data.
Individual seed regions (bilateral anterior and posterior hippocampus) were derived from the structural scan using Freesurfer 5.1 ( https://surfer.nmr.mgh.harvard.edu ) ( Fischl, 2012 ) and edited using Automatic Segmentation Adapter Tool ( www.nitrc.org/projects/segadapter ) ( Wang et al., 2011 ). The hippocampus was divided into anterior and posterior segments using manual identification of standard anatomic landmarks. The uncal apex served as the border between anterior and posterior hippocampus ( Duvernoy, 2005 ; Weiss et al., 2005 ). Raters were blind to participant age and sex. Reliability for identification of these landmarks indicated 94.60% agreement within 1 slice and 99.99% agreement within 2 slices. Intraclass correlation coefficients were high and ranged from 0.897 to 0.985. When there was disagreement between raters on the correct slice location, the more experienced rater's slice was used.
Task-free functional connectivity analyses were conducted in AFNI. First, to minimize the effect of head motion, volumes with framewise displacement (FD) ≥ 0.5 mm were scrubbed in addition to 1 volume before and 1 volume after the offending volume. All children included in the final statistical analyses had data ≥ 4.87 min in length and mean FD from 0.05 to 0.50 (0.20 ± 0.10, mean ± SD). Mean FD, absolute movement, and data length for each age group are reported in Table 2 . There was a marginally significant relation between age and mean FD for the analyses testing the age-related changes in concurrent relations using the fixed effects model (β = −0.011, SE = 0.006, t = −1.773, p = 0.077). For the two prediction analyses, independent t tests indicated that there were no differences in any motion parameters between young and old cohorts ( p > 0.70).
Sample size and motion parameters (mean, SD) of all age groups for each analysis a
Correlations between the time series of the individual seed regions (bilateral anterior and posterior hippocampus) and those of the whole brain were calculated to generate individual resting state functional connectivity maps. Subsequently, Fisher's r -to- z transformation was used to convert r maps into z maps to obtain normally distributed values of the connectivity maps. The z values were extracted from 6 ROIs: inferior/superior parietal lobule (IPL/SPL), inferior occipital gyrus (IOG), left inferior temporal gyrus (ITG), left inferior frontal gyrus (IFG), fusiform gyrus, and orbital frontal gyrus (OFG) ( Fig. 3 ). These brain regions were defined according to previous research, which indicated that 4- to 8-year-old children showed greater activation in these regions during encoding for items (i.e., pictures of animal and objects) subsequently remembered with correct sources than the ones with incorrect sources (i.e., pictures of cartoon characters) (for details, see Geng et al., 2019 ). The activation differences suggest that these six brain regions are critical for encoding contextual information. Therefore, these regions were chosen as ROIs in the current study.
ROIs used in hippocampal functional connectivity analyses. Adapted from Geng et al. (2019) .
Statistical analyses
Linear mixed models were used given their capability to handle unbalanced and incomplete longitudinal data. Specifically, a series of fixed effects models were run in SPSS 20.0 to test the associations between age, source memory, and iHFC. First, age-related changes in source memory were assessed. Then, relations between age, source memory, and iHFC to each ROI were analyzed to assess the changes in concurrent relations. Next, the models were used to examine whether earlier changes in source memory predicted later iHFC with each ROI and whether earlier functional connectivity with each ROI predicted later changes in source memory.
As suggested in the Introduction, it takes time for behavioral and brain changes to mold each other. Therefore, we analyzed the relations of memory changes between W1 and W2 to functional connectivity at W3 ( Fig. 1 ). In addition, we calculated the relations of functional connectivity at W1 to memory changes between W1 and W3 ( Fig. 1 ). Another practical reason for why the memory change intervals were different between the two prediction analyses was that there was no significant change in source memory between W2 and W3 in the old cohort. [Another question of interest would be how changes in source memory are related to changes in iHFC; however, in the current sample, there was too much data loss because of motion to examine changes in iHFC within the two cohorts. Therefore, single time point data are used for functional connectivity analyses.]
Continuous covariates (age, mean FD, source memory change, and iHFC) in these models were standardized across the full sample. Akaike's Information Criterion was used to compare models of main effects to models that included both main and interactive effects. Models with the lowest Akaike's Information Criterion value were selected as the best fitting model. Given the moderate sample size (see Table 1 ) and the limited number of ROI, corrections for multiple comparisons were not applied. If interactions involving cohort (young vs old) were observed, the relation between source memory measures and iHFC was estimated again for each cohort by restandardizing the covariates for each group separately and re-estimating model parameters.
For all linear mixed models involving iHFC as the dependent measure, bilateral hippocampal subregion (anterior vs posterior) was included as a within-subjects factor. Since differences between hippocampal subregions have been reported in a previous study ( Blankenship et al., 2017 ), results related to subregion differences are reported briefly.
Behavioral results
There were 194 children who provided data for behavioral analyses ( Table 1 ). To predict source memory performance, fixed effects models, including age, cohort (young vs old), and an age × cohort interaction had better fit than the model that only included main effects. Age was positively related to source memory performance (β = 0.051, SE = 0.023, t (353) = 2.281, p = 0.023), and there was a significant interaction between age and cohort (β = –0.075, SE = 0.031, t (353) = −2.423, p = 0.016). When this interaction was probed, results showed that age-related changes in source memory were greater in the young versus old cohort ( p < 0.001 vs p = 0.028; Fig. 4 ). No differences were observed between the 6-year-old children from the young cohort who were tested at W3 and the 6-year-old children from the old cohort who were tested at W1 ( p > 0.90). Additionally, we tested whether there were differences in source memory between age groups in each cohort. All the comparisons between age groups reached significance with the exception of the difference between 7 and 8 years ( p = 0.24).
Relations between age and memory performance for each cohort.
Age-related changes in concurrent relations between source memory and functional connectivity
Fixed effects of the linear mixed models included subregion, age, and source memory as independent variables and iHFC as the dependent variable. Mean FD was also included in the model because of the marginally significant relation between age and mean FD as reported above. The main effects models had better fit than the models with age × source memory interaction. There were significant main effects of age in predicting the connectivity between hippocampus and left IPL/SPL, IOG, left ITG, left IFG, fusiform gyrus, and OFG. Specifically, as age increased, iHFC increased as well ( Fig. 5 ). The main effect of source memory was not significant for the connectivity between hippocampus and any ROI.
Significant associations between age and iHFC with each ROI. Green represents fit line for anterior hippocampus. Blue represents fit line for posterior hippocampus.
Additionally, there were differences in the connectivity between anterior versus posterior hippocampus and fusiform, left ITG, IOG, OFG, and left IPL/SPL ( Table 3 ). In all regions except OFG, connectivity was greater for posterior compared with anterior hippocampus. In IFG, there was a trending difference in hippocampal functional connectivity (anterior < posterior; Table 3 ).
Differences in iHFC between anterior and posterior hippocampus
Predictive relations between source memory changes from W1 to W2 and functional connectivity at W3
Fixed effects of the linear mixed models included subregion (anterior vs posterior), cohort (young vs old), and source memory change (defined as the differences in source memory between W1 and W2). Mean FD was not included in the models because there was no difference in any of the motion parameters (mean FD, absolute movement, and data length) between young and old cohorts ( p > 0.60). The models containing the cohort × source memory change interaction resulted in better model fit for left IFG and OFG. In contrast, the main effects models had better fit for the other four ROIs. Source memory changes between W1 and W2 were positively related to W3 hippocampal functional connectivity with left IPL/SPL, IOG, left ITG, and fusiform gyrus ( Table 4 ; Fig. 6 ).
Relations between source memory changes from W1 to W2 and iHFC at W3 a
Plots represent the relations between source memory changes and functional connectivity for ROIs showing a main effect of source memory change.
There were significant interactions between cohort and source memory change predicting intrinsic functional connectivity from hippocampus to left IFG and OFG ( Table 4 ). To probe these interactions, the fixed effects models were used to assess each cohort separately. In the young cohort, source memory changes were significantly related to iHFC with left IFG and OFG (β = 0.109, SE = 0.025, t (73) = 4.341, p < 0.001; β = 0.068, SE = 0.022, t (73) = 3.117, p = 0.003). However, in the old cohort, these associations were not apparent ( p values > 0.20). Therefore, only in the young cohort did behavioral improvements in source memory shape later intrinsic functional connectivity between hippocampus and these cortical regions ( Fig. 7 ). Moreover, we reran the analyses above with source memory at W1 and W2 included in the models to test the relations between source memory changes and later hippocampal functional connectivity. All results stayed the same, except that the main effect of source memory changes in left IPL/SPL and IOG became nonsignificant ( p = 0.18; p = 0.11).
Plots represent associations between source memory changes and iHFC for each cohort from analyses showing significant interactive effects. * p < 0.01.
Additionally, posterior hippocampus showed greater connectivity to fusiform, left ITG, IOG, and left IPL/SPL than anterior hippocampus. The difference in OFG and left IFG did not reach significance ( Table 3 ).
Predictive relations between functional connectivity at W1 and source memory changes from W1 to W3
Fixed effects models included iHFC at W1 and cohort (young vs old) as independent variables and source memory change from W1 to W3 as the dependent variable. Mean FD was not included in the model as it was not significantly different between cohorts ( p > 0.70). For left ITG and fusiform, the interaction models had better fit. For the other ROIs, the main effects models had better fit but did not generate any significant finding.
The interactions between cohort and iHFC in left ITG and fusiform gyrus significantly predicted source memory change ( Table 5 ). To probe the interactions, fixed effects models were used to test the relations in each cohort separately. In the older cohort, iHFC with left ITG and fusiform gyrus was related to source memory change (β = –0.090, SE = 0.024, t (60) = 3.76, p < 0.001; β = 0.086, SE = 0.029, t (60) = 2.998, p = 0.004). However, this association was not observed in the young cohort ( p > 0.80). Therefore, only in the old cohort did functional connectivity between hippocampus and left ITG and fusiform gyrus predict later improvements in source memory ( Fig. 7 ).
Relations between iHFC at W1 and source memory change from W1 to W3 a
This study used an accelerated longitudinal design to investigate the predictive relations between source memory changes and iHFC across childhood. First, we found age-related increases in source memory performance, supporting previous studies highlighting age-related differences in source memory in this age group ( Drummey and Newcombe, 2002 ; Riggins, 2014 ). Second, results indicated that iHFC within the memory network increased across childhood, which is consistent with previous cross-sectional studies showing similar associations between age and hippocampal functional connectivity ( Blankenship et al., 2017 ; Geng et al., 2019 ). Finally, results indicated that changes in memory performance predicted later functional connectivity and early functional connectivity predicted later source memory changes.
Source memory changes predicted later hippocampal functional connectivity
Source memory changes early in life predicted later intrinsic connectivity between the hippocampus and cortical regions in the absence of a task, suggesting that behavior shaped later functional connectivity. Specifically, across all children, changes in memory predicted intrinsic connectivity from hippocampus to fusiform, left ITG, IOG, and left IPL/SPL 1 year later. Additionally, within the younger cohort, early memory changes also predicted hippocampal functional connectivity with left IFG and OFG. However, in analyses assessing aged-related changes in concurrent relations, memory ability was not related to iHFC. These findings support the idea that life experience shapes the brain across development, a notion that has received support from other findings as well ( Fox and Raichle, 2007 ; Gabard-Durnam et al., 2016 ). For instance, task-elicited functional connectivity has been shown to predict resting-state functional connectivity 2 years later but not concurrently ( Gabard-Durnam et al., 2016 ). Additionally, as suggested by the interactive specialization framework, we interpreted the findings to show that changes in memory early in life may drive functional integration within the hippocampal memory network and that this process takes time to unfold ( Johnson, 2011 ; Poppenk et al., 2013 ). In other words, early memory gains may reflect gradual refinement of the hippocampal memory network over time, which ultimately leads to enhanced iHFC even during the absence of a specific task. This is consistent with previous research in other cognitive domains showing that arithmetic training induced changes in hippocampal-parietal connectivity in middle childhood ( Rosenberg-Lee et al., 2018 ).
Hippocampal functional connectivity predicted later source memory changes
Connectivity from hippocampus to fusiform and ITG predicted memory changes 2 years later. However, this was only true for the older cohort, such that iHFC with fusiform and left ITF at 6 years predicted memory changes 2 years later. These findings support the claim that activity in the brain can also shape behavior, and suggest that functional integration within the hippocampal memory network can predict future developmental gains in memory ability in children as young as 6 years of age. This finding is consistent with previous studies suggesting that earlier iHFC can predict intervention-induced behavioral changes in typical and atypical developmental populations ( Hoeft et al., 2011 ; Supekar et al., 2013 ). Therefore, our study suggests that, early in life, the interaction between brain and behavioral development is not unidirectional, but instead a reciprocal process: behavioral changes shape future brain activity, and brain activity shapes future behavioral changes.
Timing effects in the interaction between behavioral and brain development
Our results suggest that timing matters in terms of the interactions between brain and behavioral development. Specifically, although earlier memory changes predicted later hippocampal connectivity with certain cortical regions in both young and old cohorts, only within the younger cohort were early memory changes related to the functional connectivity from hippocampus to left IFG and OFG. In contrast, early functional connectivity with fusiform and left ITG significantly predicted later memory changes in the older cohort only. We propose two possible explanations of these timing differences. First, these findings may result from known differences in the magnitude of age-related changes in the development of source memory during childhood. Consistent with previous behavioral studies suggesting accelerated rates of change in source memory before the sixth year of life ( Riggins, 2014 ), the current study indicates that memory changes for the 4-to-6-year old cohort were more substantial than for the 6-to-8-year old cohort. Greater memory changes may drive greater changes in the brain. This may help explain why earlier memory changes in the younger cohort predicted connectivity between the hippocampus and IFG and OFG.
Alternatively, the timing difference may result from known differences in developmental changes in the hippocampus during childhood. Studies in humans and animals consistently indicate that the hippocampal memory network experiences significant development around the sixth year of life ( Serres, 2001 ; Lavenex and Lavenex, 2013 ). Therefore, 4-6 years may be a sensitive period for hippocampal development, characterized by greater plasticity ( Tottenham and Sheridan, 2010 ). This claim is consistent with the argument that shaping functional architecture may be particularly pronounced during developmental periods when neural systems are most plastic and amenable to environmental inputs ( Bick and Nelson, 2017 ). After the sensitive period ends, the more mature hippocampal memory network may begin driving developmental changes in memory abilities. This may explain why iHFC predicted behavioral changes in the older cohort only. Therefore, we speculate that only after the brain reaches a certain threshold of maturation can its function reliably and consistently predict behavior.
The timing effects may also depend on the specific brain regions exhibiting functional connectivity with the hippocampus. Early memory changes from 4 to 5 years predicted functional connectivity between hippocampus and prefrontal regions (IFG and OFG) at 6 years. However, in the older cohort, functional connectivity at age 8 years was not predicted by behavior changes from 6 to 7 years. In contrast, only in the older cohort did iHFC predict memory changes 2 years later, and this was specific to the fusiform and ITG. These findings may be because of the different developmental trajectories of these neural regions. Previous research has shown that brain development proceeds in a hierarchical sequence from sensory to association cortices to regions important for cognitive functions ( Gogtay et al., 2004 ; Shaw et al., 2008 ). IFG and OFG are regions associated with higher-order cognition that mature later than sensory or association regions. Thus, the findings of the current study are consistent with the notion that prefrontal regions, such as IFG and OFG, are less mature, and therefore more plastic, in the younger versus older cohort. This is also consistent with previous studies indicating that increased hippocampal functional coupling with the PFC is related to the development of memory abilities ( Qin et al., 2014 ). The present findings extend this work and suggest that 4-6 years may be a sensitive period for the functional integration between hippocampus and frontal regions. In contrast, fusiform and ITG are related to visual processing and mature earlier than prefrontal regions. Such early maturation might underlie the ability of fusiform and ITG to predict behavioral changes.
Strengths and limitations
The current study has several strengths. By using an accelerated longitudinal design, we traced developmental changes in source memory, iHFC, and relations between them. This design also allowed us to test timing differences by comparing two cohorts of children. By focusing on a proposed sensitive period for the development of source memory, we were able to examine how the hippocampal memory network is refined within this important developmental window and how it relates to improvements in memory.
This study also has limitations. Because of the limited sample size, we were unable to examine how changes in functional connectivity over time relate to memory. Also, because of the sample size, we elected not to conduct whole-brain analyses and instead relied on ROIs derived from a task-based study involving children from the same longitudinal study. Thus, findings with additional brain regions, not included in the current set of ROIs, may also exist. Additionally, the ROIs were defined in a task using only pictorial material. In contrast, source memory in this study was measured in a task involving both pictorial and verbal material. It is unknown whether different brain regions would be activated to encode contextual information tapping visual versus verbal modalities. Finally, although a proportion of children in the sample provided task-based fMRI data to define ROIs, we did not have enough task-based MRI data to assess developmental relations between the task-based hippocampal functional connectivity and the connectivity measured without a demanding task imposed.
In conclusion, our study focused on the mechanisms related to developmental changes in source memory and the underlying hippocampal network between 4 and 8 years of age. Results indicate that behavioral experience and brain changes are interactive processes, such that experience shapes changes in the brain and the brain shapes changes in behavior. Additionally, our findings support and extend previous studies of brain development by showing that timing matters in terms of behavioral changes molding brain connectivity and such timing differences also depend on which brain regions are involved. Together, these findings add critical insight into the development of source memory in early childhood and contribute to the growing body of literature documenting the interactive and intricate relations between cognitive processes and their neurologic bases in the developing brain.
This work was supported by National Institute of Child Health and Human Development Grant HD079518 and the University of Maryland. We thank members of the Neurocognitive Development Lab, especially Kelsey Canada, Elizabeth Mulligan, Marissa Clark, Lisa Cox, Shane Wise, and Jennifer Sloane, for helping with data collection and/or analysis.
The authors declare no competing financial interests.
- Correspondence should be addressed to Tracy Riggins at riggins{at}umd.edu
SfN exclusive license .
- Blankenship SL ,
- Dougherty LR ,
- Cooper RA ,
- Drummey AB ,
- Newcombe NS
- Duvernoy HM
- Kochalka J ,
- Battista C ,
- Gabard-Durnam LJ ,
- Flannery J ,
- Humphreys KL ,
- Lumian DS ,
- Fareri DS ,
- Caldera C ,
- Tottenham N
- Hayashi KM ,
- Greenstein D ,
- Vaituzis AC ,
- Nugent TF ,
- Herman DH ,
- Clasen LS ,
- Rapoport JL ,
- Thompson PM
- McCandliss BD ,
- Gantman A ,
- Zakerani N ,
- Lyytinen H ,
- Whitfield-Gabrieli S ,
- Glover GH ,
- Gabrieli JDE
- Supekar K ,
- Richardson J ,
- Tenison C ,
- Ashkenazi S ,
- Rosenberg-Lee M ,
- Lavenex P ,
- Miller JF ,
- Neufang M ,
- Trippel M ,
- Kahana MJ ,
- Schulze-Bonhage A
- Poppenk J ,
- Evensmoen HR ,
- Moscovitch M ,
- Riggins T ,
- Botdorf M ,
- Canada KL ,
- Iuculano T ,
- Wendelken C ,
- Kabani NJ ,
- Eckstrand K ,
- Lenroot R ,
- Swigart AG ,
- Jolles DD ,
- Shafer AT ,
- Tottenham N ,
- Vanderwal T ,
- Eilbott J ,
- Castellanos FX
- Altinay M ,
- Yushkevich PA
In this issue

- Table of Contents
- Table of Contents (PDF)
- About the Cover
- Index by author
- Ed Board (PDF)
Thank you for sharing this Journal of Neuroscience article.
NOTE: We request your email address only to inform the recipient that it was you who recommended this article, and that it is not junk mail. We do not retain these email addresses.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
- Google Plus One
Jump to section
Responses to this article, jump to comment:, related articles, cited by..., more in this toc section, research articles.
- Striatal Serotonin Release Signals Reward Value
- Brief and Diverse Excitotoxic Insults Increase the Neuronal Nuclear Membrane Permeability in the Neonatal Brain, Resulting in Neuronal Dysfunction and Cell Death
- Cells and Molecules Underpinning Cannabis-Related Variations in Cortical Thickness during Adolescence
Development/Plasticity/Repair
- Neuritin Controls Axonal Branching in Serotonin Neurons: A Possible Mediator Involved in the Regulation of Depressive and Anxiety Behaviors via FGF Signaling
- Proper Frequency of Perinatal Retinal Waves Is Essential for the Precise Wiring of Visual Axons in Nonimage-Forming Nuclei
- Monocyte invasion into the retina restricts the regeneration of neurons from M ü ller glia
Mind & Brain News
Top headlines, latest headlines.
- Remote Brain Stimulation
- Talking About Parents in Therapy: Emotions
- A New Target for Anxiety Disorders
- Neurodevelopment: Genetic Disorders
- Near Infrared Light Treatment for Brain Injury
- Brainstem Damage: Long-Lasting COVID Symptoms
- Small Brains Can Accomplish Big Things
- Precise Brain-Circuit Control
- Guesses About the Past; Predictions of Future
- Coffee During Pregnancy: Safe for Baby's Brain?
Earlier Headlines
Friday, october 11, 2024.
- How Do We Recognize Other People's Emotions?
Thursday, October 10, 2024
- Students Who Feel More University Connection May Be More Likely to Binge Drink, Study Finds
- Bilingualism Makes the Brain More Efficient, Especially When Learned at a Young Age
- Glowing Approach Could Aid Carpal Tunnel-Related Surgery
- Heart Failure, Atrial Fibrillation and Coronary Heart Disease Linked to Cognitive Impairment
- To Make Children Better Fact-Checkers, Expose Them to More Misinformation -- With Oversight
- Simulation Mimics How the Brain Grows Neurons, Paving the Way for Future Disease Treatments
- Understanding How Smiling Influences Relationship Building During Real-Life Conversations
Wednesday, October 9, 2024
- In Studying the Mating Rituals of Fruit Flies, Scientists May Have Learned Something About How Brains Evolve
- Do People With MS Have an Increased Risk of Cancer?
- Gene Therapy Shows Long-Term Benefit for Patients With a Rare Pediatric Brain Disease
- Why People Think They're Right, Even When They Are Wrong
- Tiny Antibodies to Fight the Dangerous Effects of Opioids
- People With Dyslexia and Dyscalculia Show Less Bias, Study Shows
- Arrhythmic Hearts After Excessive Alcohol Consumption
- Neurons Look Different in Children With Autism, Research Finds
- Are Ideas Contagious?
- Toddlers Show Increased Physical Activity With a Robot Playmate Moving Around the Room
- Scientists Create Map of DNA Modification in the Developing Human Brain
- Extended Timing: How Neurons Encode Information on Timescales That Match Learning
- Another Step Towards Decoding Smell
Tuesday, October 8, 2024
- Echoes in the Brain: Why Today's Workout Could Fuel Next Week's Bright Idea
- One-Time Cooperation Decisions Unaffected by Increased Benefits to Society
- Hip Osteoarthritis: Head Gets in the Way of Recovery, Study Suggests
- Increase Access to Nature in All Daily Environments and in Education
- Mental Health App Could Help Prevent Depression in Young People at High Risk
- Recreating a Hallmark of Parkinson's Disease in Human Neurons

Monday, October 7, 2024
- Study Explores Novel Therapeutic Treatment for Glioblastoma
- Brain's Waste-Clearance Pathways Revealed
- Fear of Childbirth Is Associated With Shorter Duration of Breastfeeding
- Autobiographical Memory in the Digital Age: Our Lives in the Mirror of Our Data
- Brain Network Study Reveals Clues About Dementia's Behavior Changes
- Commonly Used Arm Positions Can Substantially Overestimate Blood Pressure Readings
- Childhood Social Interactions Can Combat Stereotypes
- Scientists Discover a Secret to Regulating Our Body Clock, Offering New Approach to End Jet Lag
Friday, October 4, 2024
- Researchers Seek to Improve Advanced Pain Management Using AI for Drug Discovery
- Real-Time Data Shows What Happens When People Lose Their Balance
- Role of Ophthalmic Acid in Motor Function Control
- Are Auditory Magic Tricks Possible for a Blind Audience?
Thursday, October 3, 2024
- Hoarding Disorder: 'Sensory CBT' Treatment Strategy Shows Promise
- Turning Brain Cells on Using the Power of Light
- What Happens in the Brain When a Person With Schizophrenia 'hears Voices'?
- Smartphone-Assisted 'scavenger Hunt' Identifies People at Risk for Dementia
- Psychological Distress in Adults and Caregivers About Food Allergy Is Widespread and Unrecognized
- Promising 'first' In Alzheimer's Drug Development
- Our Brains Divide the Day Into Chapters: New Psychology Research Offers Details on How
Wednesday, October 2, 2024
- Researchers Use AI to Help People See More Clearly
- Medical and Psychological Harms of Obesity Depend on Where You Live, Study Indicates
- How Estrogen's Millisecond-Fast Action Happens
- Satisfying Friendships Could Be Key for Young, Single Adults' Happiness
- New Cardiovascular Disease Risk Marker Discovered in Older Women
- New Study Explores How Universities Can Improve Student Well-Being
- Chronic Pain Patients Are More Supportive of Cannabis Access Than Doctors
- Neuroscience Breakthrough: Entire Brain of Adult Fruit Fly Mapped
- New Research Offers Hope for Preventing Age-Related Blindness
- Brain Molecule Makes Neurons Less Selective, Deepening Understanding of Human Cognition
- Eyes on the Fries: How Our Vision Creates a Food Trend
- For Long COVID, Lithium Aspartate at Low Doses Is Ineffective, but Higher Doses May Be Promising, Study Finds
- Clinical Trial Shows Synthetic Cannabis Reduces Agitation in Alzheimer's Disease
- Dementia Diagnostic Markers Change With Time of Day
- New Study Finds a Promising Combined Therapy for Multiple Sclerosis
- AI Simulation Gives People a Glimpse of Their Potential Future Self
- Neurological Disorder of Mirror Movements: Looking Deeper Into the Mirror
Tuesday, October 1, 2024
- Research in 4 Continents Links Outdoor Air Pollution to Differences in Children's Brains
- 'Who's a Good Boy?' Humans Use Dog-Specific Voices for Better Canine Comprehension
- Neuroscientists Spark Shelter-Seeking Response by Reactivating Memory Circuit
- New Therapeutic Approach to Preventing Cancer from Spreading to the Brain
- ChatGPT Shows Human-Level Assessment of Brain Tumor MRI Reports
- Lab-Grown Spines Unlock Safer Treatment for Women With Epilepsy, Study Suggests
- Feet First: AI Reveals How Infants Connect With Their World
- Researchers Integrate Fast OCT System Into Neurosurgical Microscope
Monday, September 30, 2024
- Study Suggests Simple Steps May Improve Team Ethics
- Alzheimer's Genetic Risk Factors Spark Inflammation in Females
- New Brain-Mapping Tool May Be the 'START' Of Next-Generation Therapeutics
- When Detecting Depression, the Eyes Have It
- New Laser-Based Headset Can Measure Blood Flow, Assess Risk of Stroke
- Circadian Disruption, Gut Microbiome Changes Linked to Colorectal Cancer Progression
Friday, September 27, 2024
- Unraveling the Role of tRNA Modifying Enzyme in Brain Function
- New Study Suggest Treatments That Maintain the Health of Synapses May Help Prevent, Mitigate the Symptoms of Prion Disease
- Sharing Biosignals With Online Gaming Partners to Enhance a Mutual Sense of Social Presence Between Complete Strangers
- Automatic Speech Recognition Learned to Understand People With Parkinson's Disease -- By Listening to Them
- Commonly Used Drug Could Transform Treatment of Rare Muscle Disorder
- Researchers Discover What Hinders DNA Repair in Patients With Huntington's Disease
- How Are Pronouns Processed in the Memory-Region of Our Brain?
Thursday, September 26, 2024
- How Social Structure Influences the Way People Share Money
- Unexpected Role of Hippocampus in Skilled Movement Control
- Bacteria Involved in Gum Disease Linked to Increased Risk of Head and Neck Cancer
- A Method of 'look Twice, Forgive Once' Can Sustain Social Cooperation
- How Are Stretch Reflexes Modulated During Voluntary Movement?
- People With Unmedicated Mental Illness Are Less Likely to Be Vaccinated Against COVID-19, Swedish Study Finds
- Cognitive Deficits from Meth and PCP Use Are Generated by a Common Neurotransmitter Switch
- Programming Cells to Target Brain Tumors
- Unlocking the Energy Crisis in Parkinson's: New Findings Offer Hope for Future Treatment
- Recording the Cats in the Hats
- Pregnant Women Who Sleep Less Than 7 Hours a Night May Have Children With Developmental Delays
- Light at the End of the Tunnel for Night Shift Workers
- Why Children With Down Syndrome Have Higher Risk of Leukemia
Wednesday, September 25, 2024
- Study Finds Certain MS Therapies May Not Slow Disability Progression
- When a Child Hurts, Validating Their Pain May Be the Best First Aid
- How Synchronization Supports Social Interactions
- LATEST NEWS
- Top Science
- Top Physical/Tech
- Top Environment
- Top Society/Education
- Health & Medicine
- Mind & Brain
- Disorders and Syndromes
- ADD and ADHD
- Alzheimer's
- Bipolar Disorder
- Borderline Personality Disorder
- Brain Injury
- Hearing Impairment
- Huntington's Disease
- Mad Cow Disease
- Multiple Sclerosis
- Obstructive Sleep Apnea
- Parkinson's
- Schizophrenia
- Sleep Disorders
- Education & Learning
- Brain-Computer Interfaces
- Educational Psychology
- Infant and Preschool Learning
- Intelligence
- K-12 Education
- Language Acquisition
- Learning Disorders
- Illegal Drugs
- Crystal Meth
- Psychedelic Drugs
- Living Well
- Anger Management
- Child Development
- Consumer Behavior
- Dieting and Weight Control
- Gender Difference
- Nutrition Research
- Racial Issues
- Relationships
- Spirituality
- Mental Health
- Eating Disorders
- Smoking Addiction
- Neuroscience
- Child Psychology
- Social Psychology
- Space & Time
- Matter & Energy
- Computers & Math
- Plants & Animals
- Earth & Climate
- Fossils & Ruins
- Science & Society
- Business & Industry
Strange & Offbeat
- Liftoff! NASA's Europa Clipper
- Mysterious Heating of the Sun's Atmosphere
- Evolution in Real Time
- High-Speed Printable Circuits: Next-Gen Displays
- Single-Atom Editing Tech Developed
- Electron 'Pinball' in Space After Lightning
- Playing Songs to Darwin's Finches: New Species
- New Tech Improves Structural Strength
- 3D Printing Method: Unique Objects Quickly
Trending Topics

Neuroscience News Home
Neuroscience News is an independent open access science magazine. Since 2001, we have featured neuroscience research news from labs, universities, hospitals and news departments around the world. Topics include brain research, AI, psychology, neuroscience, mental health and neurotech.
Latest Neuroscience News
Science news articles cover neuroscience, neurology, psychology, AI, mental health, robotics, neurotechnology and cognitive sciences.

Key Protein Regulates DNA Enzymes, Supporting Genome Stability

Imaging Links Fewer Brain Connections to Autism Social Challenges

25% of Adults Suspect Undiagnosed ADHD
Neurology news articles.
Neurology news articles cover neurology, brain cancer , traumatic brain injuries , neurosurgery, neuroanatomy, brain research and neurological disorders.

Infrared Light Therapy Shows Promise for Brain Injury Recovery
Persistent Virus May Drive Long COVID

Liver Inflammation Linked to Cognitive Decline in Aging
Ai news articles.
AI news articles cover science articles about artificial intelligence including ChatGPT, Bard, Dalle, neural networks, machine learning, LLMs, AGI and other AI related topics.

From Face to Feeling: Context Shapes Emotion Recognition

Can AI Tackle Abstract Reasoning? Study Tests Cognitive Limits

Electronic Tongue Uses AI to Detect Differences in Liquids
Psychology news articles.
Science research articles cover psychology, depression, mental health , schizophrenia, mental disorders, happiness, stress, PTSD, autism, psychiatry and therapy.

When Dogs and Humans Connect, So Do Their Brainwaves

Teen Friendships Shape Long-Term Wellbeing

Reflecting on Parents in Therapy Can Shift Childhood Memories
Trending neuroscience news.
These are the most viewed Neuroscience News articles of the month.
Key Brain Protein Tied to Motivation and Mood Identified
Gut Microbiome and Aspirin May Reverse Hormonal Issues
Targeting Glucose May Spark Neurogenesis

Child Trauma Recovery Tied to Thoughts, Not Event Severity

Screen Time Linked to Mental Health Symptoms in Kids
Humans Can Detect Quick Chemical Changes in Smells with Each Sniff

Human-Specific Genes Reveal Link Between Brain Growth and Autism

- Israel-Gaza War
- War in Ukraine
- US & Canada
- UK Politics
- N. Ireland Politics
- Scotland Politics
- Wales Politics
- Latin America
- Middle East
- In Pictures
- BBC InDepth
- US Election
- Election polls
- Kamala Harris
- Donald Trump
- Executive Lounge
- Technology of Business
- Women at the Helm
- Future of Business
- Science & Health
- Artificial Intelligence
- AI v the Mind
- Film & TV
- Art & Design
- Entertainment News
- Arts in Motion
- Destinations
- Australia and Pacific
- Caribbean & Bermuda
- Central America
- North America
- South America
- World’s Table
- Culture & Experiences
- The SpeciaList
- Natural Wonders
- Weather & Science
- Climate Solutions
- Sustainable Business
- Green Living
Fly brain breakthrough 'huge leap' to unlock human mind

They can walk, hover and the males can even sing love songs to woo mates - all this with a brain that’s tinier than a pinhead.
Now for the first time scientists researching the brain of a fly have identified the position, shape and connections of every single one of its 130,000 cells and 50 million connections.
It's the most detailed analysis of the brain of an adult animal ever produced.
One leading brain specialist independent of the new research described the breakthrough as a "huge leap" in our understanding of our own brains.
One of the research leaders said it would shed new light into “the mechanism of thought”.
Dr Gregory Jefferis, of the Medical Research Council's Laboratory of Molecular Biology (LMB) in Cambridge told BBC News that currently we have no idea how the network of brain cells in each of our heads enables us to interact with each other and the world around us.
“What are the connections? How do the signals flow through the system that can let us process the information to recognise your face, that lets you hear my voice and turn these words into electrical signals?
“The mapping of the fly brain is really remarkable and will help us get a real grasp of how our own brains work.”
We have a million times as many brain cells, or neurons, than the fruit fly which was studied. So how can the wiring diagram of an insect brain help scientists learn how we think?
The images the scientists have produced, which have been published in the journal Nature , show a tangle of wiring that is as beautiful as it is complex.
Its shape and structure holds the key to explaining how such a tiny organ can carry out so many powerful computational tasks. Developing a computer the size of a poppy seed capable of all these tasks is way beyond the ability of modern science.
Dr Mala Murthy, another of the project’s co-leaders, from Princeton University, said the new wiring diagram, known scientifically as a connectome, would be “transformative for neuroscientists”.
“It will help researchers trying to better understand how a healthy brain works. In the future we hope that it will be possible to compare what happens when things go wrong in our brains.”
That is a view backed by Dr Lucia Prieto Godino, a group leader in brain research at the Francis Crick Institute in London, who is independent of the research team.
"Researchers have completed the connectomes of a simple worm which has 300 wires and a maggot which has three thousand, but having a complete connectome of something with 130,000 wires is an amazing technical feat which paves the way for finding the connectomes for larger brains such as the mouse and maybe in several decades our own."
The researchers have been able to identify separate circuits for many individual functions and show how they are connected.
The wires involved with movement for example are at the base of the brain, whereas those for processing vision are towards the side. There are many more neurons involved in the latter because seeing requires much more computational power.
While scientists already knew about the separate circuits they did not know how they were connected together.
Why are flies so difficult to swat?
Other researchers are already using the circuit diagrams, for example to work out why flies are so difficult to swat.
The vision circuits detect which direction your rolled up newspaper is coming from, and they pass on the signal to the fly's legs.
But crucially, they send a stronger jumping signal to the legs facing away from the object of their imminent demise. So you could say they jump away without even having to think – literally faster than the speed of thought.
This finding may explain why we lumbering humans seldom squash flies.

The wiring diagram was made by slicing up a fly brain using what is essentially a microscopic cheese grater, photographing each of the 7,000 slices and digitally putting them altogether. Then the Princeton team applied artificial intelligence to extract the shapes and connections of all the neurons. But the AI wasn’t perfect – the researchers still had to fix over three million mistakes by hand.
This in itself was a technical tour de force, but the job was only half done. The map on its own was meaningless unless there was a description of what each wire was supposed to do, according to Dr Philipp Schlegel, who is also from the Medical Research Council's Laboratory of Molecular Biology.
“This data is a bit like Google Maps but for brains: the raw wiring diagram between neurons is like knowing which structures correspond to streets and buildings.
"Describing the neurons is like adding the names for streets and towns, business opening times, phone numbers, reviews, etc. to the map. You need both for it to be really useful.”
The fly connectome is available to any scientist that wants to use it to guide their research. Dr Schlegel believes that the world of neuroscience will see “an avalanche of discoveries in the next couple of years” thanks to this new map.
A human brain is so much larger than the fly’s, and we don’t yet have the technology to capture all the information about its wiring.
But the researchers believe that perhaps in 30 years it may be possible to have a human connectome. The fly brain, they say, is a start of a new, deeper understanding of how our own minds work.
The research has been conducted by a large international collaboration of scientists, called the FlyWire Consortium.
The human brain, explained
Learn about the most complex organ in the human body, from its structure to its most common disorders.
Here’s something to wrap your mind around: The human brain is more complex than any other known structure in the universe . Weighing in at three pounds, on average, this spongy mass of fat and protein is made up of two overarching types of cells—called glia and neurons—and it contains many billions of each. Neurons are notable for their branch-like projections called axons and dendrites, which gather and transmit electrochemical signals. Different types of glial cells provide physical protection to neurons and help keep them, and the brain, healthy.
Together, this complex network of cells gives rise to every aspect of our shared humanity. We could not breathe, play, love, or remember without the brain.
Anatomy of the brain
The cerebrum is the largest part of the brain , accounting for 85 percent of the organ's weight. The distinctive, deeply wrinkled outer surface is the cerebral cortex. It's the cerebrum that makes the human brain—and therefore humans—so formidable. Animals such as elephants, dolphins, and whales actually have larger brains, but humans have the most developed cerebrum. It's packed to capacity inside our skulls, with deep folds that cleverly maximize the total surface area of the cortex .
The cerebrum has two halves, or hemispheres, that are further divided into four regions, or lobes. The frontal lobes, located behind the forehead, are involved with speech, thought, learning, emotion, and movement. Behind them are the parietal lobes, which process sensory information such as touch, temperature, and pain. At the rear of the brain are the occipital lobes, dealing with vision. Lastly, there are the temporal lobes, near the temples, which are involved with hearing and memory.
The second-largest part of the brain is the cerebellum , which sits beneath the back of the cerebrum. It plays an important role in coordinating movement, posture, and balance.
The third-largest part is the diencephalon, located in the core of the brain. A complex of structures roughly the size of an apricot, its two major sections are the thalamus and hypothalamus. The thalamus acts as a relay station for incoming nerve impulses from around the body that are then forwarded to the appropriate brain region for processing. The hypothalamus controls hormone secretions from the nearby pituitary gland. These hormones govern growth and instinctual behaviors, such as when a new mother starts to lactate. The hypothalamus is also important for keeping bodily processes like temperature, hunger, and thirst balanced.
YEAR-LONG ADVENTURE for every young explorer on your list
FREE limited-edition frog drawstring bag with every Nat Geo Kids Book Bundle subscription
Seated at the organ's base, the brain stem controls reflexes and basic life functions such as heart rate, breathing, and blood pressure. It also regulates when you feel sleepy or awake and connects the cerebrum and cerebellum to the spinal cord.

The brain is extremely sensitive and delicate, and so it requires maximum protection, which is provided by the hard bone of the skull and three tough membranes called meninges. The spaces between these membranes are filled with fluid that cushions the brain and keeps it from being damaged by contact with the inside of the skull.
Blood-brain barrier
Want more proof that the brain is extraordinary? Look no further than the blood-brain barrier. The discovery of this unique feature dates to the 19th century, when various experiments revealed that dye, when injected into the bloodstream, colored all of the body’s organs except the brain and spinal cord. The same dye, when injected into the spinal fluid, tinted only the brain and spinal cord.
You May Also Like

Can positive thinking prolong your life? Science says yes

How to get high on your own hormones—naturally

Can you really sweat out toxins?
This led scientists to learn that the brain has an ingenious, protective layer. Called the blood-brain barrier, it’s made up of special, tightly bound cells that together function as a kind of semi-permeable gate throughout most of the organ . It keeps the brain environment safe and stable by preventing some toxins, pathogens, and other harmful substances from entering the brain through the bloodstream, while simultaneously allowing oxygen and vital nutrients to pass through.
Health conditions of the brain
Of course, when a machine as finely calibrated and complex as the brain gets injured or malfunctions, problems arise. One in five Americans suffers from some form of neurological damage , a wide-ranging list that includes stroke, epilepsy, and cerebral palsy, as well as dementia.
Alzheimer’s disease , which is characterized in part by a gradual progression of short-term memory loss, disorientation, and mood swings, is the most common cause of dementia . It is the sixth leading cause of death in the United States, and the number of people diagnosed with it is growing. Worldwide, some 50 million people suffer from Alzheimer’s or some form of dementia. While there are a handful of drugs available to mitigate Alzheimer’s symptoms, there is no cure. Researchers across the globe continue to develop treatments that one day might put an end to the disease’s devasting effects.
Far more common than neurological disorders, however, are conditions that fall under a broad category called mental illness . Unfortunately, negative attitudes toward people who suffer from mental illness are widespread. The stigma attached to mental illness can create feelings of shame, embarrassment, and rejection, causing many people to suffer in silence. In the United States, where anxiety disorders are the most common forms of mental illness, only about 40 percent of sufferers receive treatment. Anxiety disorders often stem from abnormalities in the brain’s hippocampus and prefrontal cortex.
Attention-deficit/hyperactivity disorder, or ADHD , is a mental health condition that also affects adults but is far more often diagnosed in children. ADHD is characterized by hyperactivity and an inability to stay focused. While the exact cause of ADHD has not yet been determined, scientists believe that it may be linked to several factors, among them genetics or brain injury. Treatment for ADHD may include psychotherapy as well as medications. The latter can help by increasing the brain chemicals dopamine and norepinephrine, which are vital to thinking and focusing.
Depression is another common mental health condition. It is the leading cause of disability worldwide and is often accompanied by anxiety. Depression can be marked by an array of symptoms, including persistent sadness, irritability, and changes in appetite. The good news is that in general, anxiety and depression are highly treatable through various medications—which help the brain use certain chemicals more efficiently—and through forms of therapy.
Related Topics
- NEUROSCIENCE

The 11 most astonishing scientific discoveries of 2023

How much of a role does genetics play in obesity?

This is why getting scared can feel so good

Why do some say natural deodorants are better—and are the claims accurate?

Unweaving the history of friendship bracelets
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Five simple questions can help spot exaggerated research claims over sex differences in the brain
Professor Emeritus of Cognitive NeuroImaging, Aston University
Disclosure statement
Gina Rippon does not work for, consult, own shares in or receive funding from any company or organization that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
Aston University provides funding as a member of The Conversation UK.
View all partners
In the last ten years, some 20,000 or so academic papers have been published on the neuroscience of sex and gender. Perhaps you have read the media coverage of such papers, suggesting there’s finally proof that stereotypical abilities such as men being good at reading maps or women excelling at nurturing can be pinpointed in the brain.
Given the sheer quantity of output in this area, how can you tell what is really groundbreaking research, and what is an overenthusiastic application of hype?
Misleading spin is often blamed on university PR teams, non-specialist science writers in mainstream newspapers, or social media. But the source of deceptive impressions may sometimes be the research papers themselves.
For example, researchers may hyper-focus on a limited set of findings. They may fail to report that many of the differences they were looking for didn’t make the statistical cut. Or they may be less than cautious in discussing the impact of their findings.
Just as much as researchers need to be meticulous about the best methodology and the most powerful statistics, they need to manage the impressions they make when communicating their research. And, if they don’t, then the interested but non-expert reader may need help to spot this.
Magic: spotting the spin
My colleagues and I recently published a set of guidelines which offer just such assistance, identifying five sources of potential misrepresentation to look out for. The initials helpfully form the acronym “Magic”, which is short for magnitude, accuracy, generalisability, inflation and credibility.
For magnitude, the question is: is the extent of any differences clearly and accurately described? Take this 2015 study on sex differences in the human brain. It reported on 34,716 different patterns of functional brain connectivity, and found statistical differences between females and males in 178 of them.
Yet given that less than 0.5% of all possible differences they were measuring actually turned out to be statistically significant, they wouldn’t really be justified in reporting sex differences as prominent. In this study, they weren’t.
The next question is to do with accuracy. Are techniques and variables clearly defined and carefully used in the interpretation of results? It should be really clear how the study was run, what measures were taken, and why.
For example, a recent paper suggesting that the Covid lockdown effects had a more pronounced effect on adolescent girls’ brain structure than boys’ fell at this hurdle. The abstract referred to “longitudinal measures” and much of the narrative was couched in longitudinal “pre- and post-Covid” terms. Longitudinal studies –– which follow the same group of people over time –– are great as they can discover crucial changes in them.
But if you peer closely at the paper, it emerges that the pre- and post-Covid lockdown comparisons appear to be between two different samples – admittedly selected from an ongoing longitudinal study. Nonetheless, it is not clear that like was compared with like.

The third question has to do with generalisability. Are authors cautious about how widely the results might be applied? Here we encounter the problem with many scientific studies being carried out on carefully selected and screened groups of participants – sometimes just their own students.
Care should be taken to ensure this is clear to the reader, who shouldn’t be left with the impression that one or more sets of participants can be taken to be fully representative of (say) all females or all males. If all study participants are selected from the same single community, then referring to “hundreds of millions of people” in interpreting the relevance of the results is something of an overstatement.
The fourth category, inflation, is to do with whether the authors avoid language that overstates the importance of their results. Terms such as “profound” and “fundamental” may be misplaced, for instance. Remember, James Watson and Francis Crick merely described their discovery of DNA’s double helix structure as of “ considerable biological interest ”.
Finally, we should consider credibility: are authors careful to acknowledge how their findings do or do not fit with existing research? Authors should be up front about alternative explanations for their findings, or suggest other factors that might need to be investigated in further studies.
Suppose, for example, they are looking at the allegedly robust sex differences in visuospatial skills, which include things like visual perception and spatial awareness. Have the authors acknowledged research suggesting that the amount of time people spend on practising this skill, such as when playing video games, has been shown to be more significant than biological sex in determining such differences?
If gamers are more likely to be boys, that doesn’t necessarily mean their brains are wired for them – it could equally well be reflecting gendered pressures that make such games a popular, culturally comfortable pastime among boys.
The focus of these guidelines is on sex/gender brain imaging studies, but they could well be applied to other areas of research.
Post-lockdown surveys have suggested that the public has greater trust in what scientists are saying than they did before the pandemic. Scientists need to be careful that they retain that trust by ensuring that what they report is unambiguous and free from hype.
Hopefully the Magic guidelines will help them and their editors achieve this; if they don’t, then eagle-eyed readers, Magic-ally armed, will be on their guard.
- Science communication
- Neuroscience
- Sex differences
- Give me perspective
Want to write?
Write an article and join a growing community of more than 191,400 academics and researchers from 5,063 institutions.
Register now
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 06 October 2021
Neuroscientists make strides towards deciphering the human brain
You have full access to this article via your institution.

Former US president Barack Obama at the 2013 launch of the BRAIN Initiative with Francis Collins, director of the National Institutes of Health. Credit: Jason Reed/Reuters/Alamy
In 2013, then-US president Barack Obama launched a US$5-billion project to improve our understanding of the human brain. The venture would take advantage of new techniques to probe the brain’s genetics and physiology. This week, Nature reports some of the results from the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative.
Although medical science continues to progress, the underlying causes of many brain disorders are not well understood at the cellular level. By the time the BRAIN Initiative ends in 2026, those involved say, it will have created a ‘gold mine’ for clinical researchers working on psychiatric, neurodegenerative and neurodevelopmental disorders.
The BRAIN Initiative Cell Census Network—Motor Cortex
The lines of evidence described in the current papers are from the BRAIN Initiative Cell Census Network (BICCN). This work is expected to help scientists to identify suitable animal models of human brain conditions — such as Parkinson’s disease, motor neuron disease and Alzheimer’s disease — that share cellular characteristics.
The BICCN project’s findings also help to explain how neurons and brain circuits are involved in emotion, behaviour and learning. These are early steps towards a more complete understanding of the neural underpinnings of human cognitive abilities — such as language and reasoning — and will keep scientists busy for decades to come.
The BRAIN Initiative is a collaboration between hundreds of researchers around the world. The BICCN project’s foundational insights include a comparison of the cells of the primary motor cortex in three species: mice, marmosets and humans 1 . The primary motor cortex is the part of the brain responsible for skilled movements, and the findings will help to reveal which cellular mechanisms are conserved across species. This will aid researchers in establishing the most appropriate model organism for studying neurodegenerative conditions.
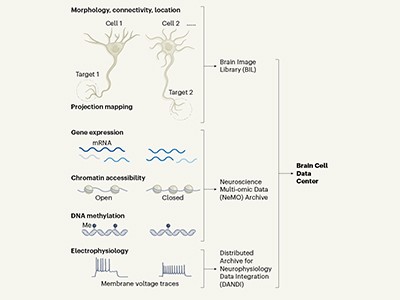
A census of cell types in the brain’s motor cortex
Scientists have also created an atlas that reveals the locations of around 25 subclasses of cell in the primary motor cortex of the same three species 1 , 2 . The researchers report what neuroscientists call an input–output wiring diagram of this region in mice. This details all of the long-distance neural connections, known as axons, reaching into and out of this region; this will help neuroscientists in their investigations of how the brain exercises motor control 3 .
Researchers have also gained insight into how cells in the human neocortex — the thin outer layers of the two cerebral hemispheres — acquire their identities during embryonic development 4 . And the project is providing scientists with tools with which to visualize and mine vast new data sets. In addition, researchers have started to create a genetic ‘toolbox’ that exploits characteristic differences in gene expression in particular cell types to label and manipulate those cells 5 . In just a few years, scientists expect to be able to peruse an online atlas charting the type and location of every cell in the mouse brain. All of the data will be freely available.
However, it’s a big leap from creating what the researchers call a cell census to understanding the precise information that a particular network of neurons is processing. Scientists do not yet know how the brain processes the streams of sensory information that tell us that we’re hungry or cold, or that create a lifetime of memories.

How the world’s biggest brain maps could transform neuroscience
Some neuroscientists think they will be able to crack the basis of these computations by breaking them down into their individual physiological and behavioural components. Others are pinning their hopes on a universal theory of brain function. One such theory, called active inference, visualizes the brain using predictive models to regulate physiology and behaviour 6 . It relies on a processing hierarchy with predictions flowing in one direction and prediction errors reported back in the opposite direction.
The BICCN researchers provide some of the tools needed to test the theory, including those required to identify and manipulate the cells that might be involved in such a circuit. But one of the experimental challenges will be to combine these cell-level tools with models of a particular aspect of perception, cognition or behaviour in live animals. Another challenge is determining the extent to which animal models might reveal useful insights about the human brain.
The BRAIN Initiative has already revealed a high degree of evolutionary conservation between the basic cellular components of the brain in different mammals. This is not surprising, given the extensive genetic overlap and similarities between species in behaviours such as eating and reproduction. But it is also reassuring, given the challenges we are already aware of regarding the extent to which animal models provide useful insights about the human brain. Whereas the mouse brain hosts around 70 million neurons, the human brain boasts some 86 billion, each one bristling with synapses, which allow them to connect to other cells. Many neurons have thousands of synaptic connections.
This difference in scale is among the reasons that Hongkui Zeng, an author of a number of the papers in this collection and director of the Allen Institute for Brain Science in Seattle, Washington, says it will take at least 50 years to create even a crude wiring diagram of a typical human brain. But as the papers published today show, scientists are making important inroads in deciphering the brain and creating tools that will one day unlock the secrets of our uniquely human cognitive attributes.
Nature 598 , 7 (2021)
doi: https://doi.org/10.1038/d41586-021-02660-x
Bakken, T. E. et al. Nature 598 , 111–119 (2021).
Article Google Scholar
BRAIN Initiative Cell Census Network (BICCN). Nature 598 , 86–102 (2021).
Muñoz-Castañeda, R. et al. Nature 598 , 159–166 (2021).
Bhaduri, A. et al. Nature 598 , 200–204 (2021).
Matho, K. S. et al. Nature 598 , 182–187 (2021).
Pezzulo, G., Rigoli, F. & Friston, K. Prog. Neurobiol. 134 , 17–35 (2015).
Article PubMed Google Scholar
Download references
Reprints and permissions
Related Articles

- Neuroscience
- Medical research
Olfactory neurons selectively respond to related visual and verbal cues
News & Views 09 OCT 24

High-performers and specialists in neuroscience research
Nature Index 02 OCT 24

Neurotechnology race ramps up, but fundamental questions remain

A modular circuit coordinates the diversification of courtship strategies
Article 09 OCT 24
Single-neuron representations of odours in the human brain

Francisco Lopera obituary: neurologist who traced genetic origin of early-onset Alzheimer’s
Obituary 11 OCT 24
World-first therapy using donor cells sends autoimmune diseases into remission
News 04 OCT 24

The global imbalance of neurological conditions
Publisher - Chemistry
London (Greater) (GB)
In-house Senior Editor
Scientist for epigenetic basis of pediatric brain disease - jamy peng lab.
Memphis, Tennessee (US)
St. Jude Children's Research Hospital (St. Jude)
Postdoctoral Research Associate - Immunology
Senior researcher - talbot laboratory.
Senior Researcher - Talbot Laboratory, Department of Surgery and Bone Marrow Transplantation and Cell Therapy
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Proc Natl Acad Sci U S A
- v.119(44); 2022 Nov 1

Sleep, Brain, and Cognition
The functions of sleep: a cognitive neuroscience perspective, katharine c. simon.
a Department of Psychology, University of California, Irvine, CA 92697;
b Psychology and Cognitive Science, University of Arizona, Tucson, AZ 85721;
Jessica D. Payne
c Department of Psychology, University of Notre Dame, Notre Dame, IN 46556
Author contributions: K.C.S., L.N., and J.D.P. wrote the paper.
This Special Feature explores the various purposes served by sleep, describing current attempts to understand how the many functions of sleep are instantiated in neural circuits and cognitive structures. Our feature reflects current experts' opinions about, and insights into, the dynamic processes of sleep. In the last few decades, technological advances have supported the updated view that sleep plays an active role in both cognition and health. However, these roles are far from understood. This collection of articles evaluates the dynamic nature of sleep, how it evolves across the lifespan, becomes a competitive arena for memory systems through the influence of the autonomic system, supports the consolidation and integration of new memories, and how lucid dreams might originate. This set of papers highlights new approaches and insights that will lay the groundwork to eventually understand the full range of functions supported by sleep.
Humans spend roughly one-third of their lives sleeping, and other animals sleep even more ( 1 , 2 ). Despite how much time is spent in this offline state, why we sleep remains a mystery. There are various candidate answers related to the immune system, hormonal systems, thermoregulatory systems, and basic metabolic processes, as sleep is essential for all of these bodily functions. Although the entire body benefits from sleep ( 3 ), the most immediate, detrimental, and unavoidable consequences of sleep loss impact the brain and the various cognitive functions it supports ( 2 , 4 ). This insight has led some researchers to conclude that “sleep is of the brain, by the brain, and for the brain” ( 5 ). This PNAS Special Feature addresses the topic of sleep from a cognitive neuroscience perspective, one that should be of broad interest given the necessity of sleep in our lives.
One link between sleep and the brain concerns the processes by which newly acquired information is stored. The notion that sleep benefits memory dates back to Ebbinghaus ( 6 ), who pioneered the experimental study of memory by demonstrating the effect of time on forgetting. He observed that forgetting occurs rapidly in the first hours after learning but progresses more slowly over the days that follow. Ebbinghaus ( 6 ) observed that forgetting seemed to slow or perhaps stop altogether between 8.8 hours postlearning and a day later. While only 2.1% of information was forgotten across this 15.2-hour period, three times that amount was forgotten over the next 24 hours. He noted that much of this period was occupied by sleep, but 40 years passed before Jenkins and Dallenbach ( 7 ) experimentally confirmed that memory retention following sleep was superior to retention following an equivalent interval of wake. Jenkins and Dallenbach ( 7 ) explained this finding by suggesting that sleep passively protects newly forming memories from the impact of interfering information. That sleep passively shelters memories against interference seems clear, but as the papers in this Special Feature demonstrate, sleep does much more for memory. It also appears to actively strengthen and shape memories as they undergo consolidation—a time-dependent process that helps to stabilize memories in brain circuits. Since the seminal research by Ebbinghaus ( 6 ) and then, Jenkins and Dallenbach ( 7 ), dozens of studies have reported that sleep benefits consolidation of memories about our daily experiences, termed episodic memory, known to be dependent on the hippocampus (e.g., ref. 7 )—especially when compared with a period of wake (refs. 7 – 9 have reviews). The putative mechanisms by which sleep influences memory consolidation are actively being explored (e.g., ref. 11 ), and the articles in this collection offer new insights into the essential nature of the sleep–memory connection.
Sleep across the Life Span
One pressing question about the sleep–memory link concerns how it manifests over one’s lifetime. Spencer and Riggins ( 10 ) examined this link at the younger end of the age spectrum. They review evidence that naps in early childhood are essential for memory consolidation, presenting a fascinating new hypothesis connecting the psychological, physiological, and neurobiological changes that accompany “nap transitions” in early childhood. Transitioning from multiple bouts of sleep each day (i.e., naps) to a single bout of overnight sleep is universal in human development, but why and exactly when this transition occurs remain unknown. Spencer and Riggins ( 10 ) argue that as the hippocampal-dependent episodic memory network matures, more efficient memory storage becomes possible. This, in turn, reduces the buildup of the pressure to sleep in the brain, known as sleep homeostasis ( 12 ), eventually enabling a young child to abandon naps in favor of consolidated overnight sleep. This hypothesis has interesting implications for how sleep supports cognitive development during the early years of life.
Denis et al. ( 13 ) extend this life span analysis to include individuals from young adulthood through middle age (18 to 59 years) using a large sample of participants spanning various backgrounds. Denis et al. ( 13 ) confirmed the importance of the sleep–memory connection across the life span, demonstrating that sleep selectively benefits memory for negative emotional information at the expense of memory for neutral information ( 14 ). This “emotional memory trade-off effect” was observed in both young and middle-aged adults, with sleep’s benefit selective to negative (but not positive) emotional memories. This work begins to address a gap in our knowledge about sleep and cognition in middle-aged adults.
Sleep within the Brain and How It Is Influenced by the Body
Guthrie et al. ( 15 ) provide a bold new insight into human sleep—replicating animal research—concerning the various stages of sleep. Basically, sleep throughout the night can be subdivided into epochs of “rapid eye movement” (REM) sleep and “nonrapid eye movement” (NREM) sleep, defined as the terms suggest by the presence or absence of eye movements, respectively. These epochs alternate over the typical night’s sleep, with NREM sleep being most prominent early in the night and REM sleep being most prominent later on. Guthrie et al. ( 15 ) confirm that two regions critical for memory—the hippocampus and cortex—can simultaneously be in different stages of sleep. In evaluating the sleep data from eight patients with both intracranial and scalp electrodes, the authors found that overall, the cortex and hippocampus spent more time in divergent than congruent states of sleep. Interestingly, different patterns emerge in the divergent, contrasting stages of sleep. The cortex appears to spend more time in wake and REM compared with the hippocampus, yet the amount of time spent in various phases of NREM appeared equivalent across the two brain regions. The findings of Guthrie et al. ( 15 ) have important functional implications for sleep-dependent cognition mechanisms during congruent and divergent states and are sure to invigorate the field of sleep research.
Chen, Zhang et al. ( 16 ) provide novel insights into the overlooked influence of the autonomic nervous system (ANS) on sleep-dependent memory mechanisms. The ANS shows clear physiologic shifts across wake and sleep stages and has recently been implicated in sleep-dependent cognition ( 17 ). In their review of the literature, these authors provide robust evidence for two distinct ANS–central nervous system networks, in which electrophysiologic features and changes in heart rate beats, known as heart rate variability, are interconnected and facilitate either the consolidation of episodic memory or performance gains in working memory. Their slow oscillation switch model highlights the competitive trade-off between these two functional networks for the limited resources available during NREM sleep and how either episodic memory or working memory domains could gain or lose performance benefits. Given the ANS life span–associated changes, their model provides new directions and testable hypotheses for sleep-dependent cognition during early and later life.
Mechanisms Underlying the Sleep–Memory Connection
Sleep, as we have seen, contributes to the storage and consolidation of memories, and developmental changes in memory storage needs may, in turn, have an impact on sleep. The “active systems consolidation” hypothesis ( 18 ) offers an integrative account of the role of sleep in memory, arguing that memory representations are repeatedly reactivated and reorganized across large-scale neuronal networks during sleep. The hippocampus is thought to orchestrate this process, which stabilizes some memories and transforms others. According to this view, the neocortex serves to integrate related and overlapping memory traces, yielding abstract representations that can be flexibly and efficiently used for the purposes of generalization and adaptive future forecasting ( 11 , 19 ). This view of sleep dovetails with modern views of memory, which hold that medial temporal lobe (MTL) regions, including the hippocampus, are at least as important for predicting the future as they are for recollecting the past ( 20 , 21 ); these regions comprise part of the default mode network (DMN), a set of brain regions active when individuals are not engaged in specific experimenter-defined tasks ( 22 ) but rather, in mental activities, such as reminiscing, future thinking, and generally constructing scenarios that help make sense of the world—all of which involve memory ( 23 ).
There remains some debate about how long the hippocampus is needed to support a fully consolidated episodic memory ( 24 , 25 ), with the active systems consolidation hypothesis favoring the view that a literal “transfer” of memory from the hippocampus to the neocortex occurs during consolidation (e.g., refs. 11 and 18 ). According to this view, episodic memories lose their dependence on the hippocampus over time and with the intervention of sleep.
Vanasse et al. ( 26 ) tested this idea using the publicly available Natural Scenes Dataset to examine memory recognition in eight human subjects on a weekly basis over the course of a year. This study deployed high-resolution (7-tesla) functional MRI to take an unprecedented look at memory consolidation as it unfolds over time. The authors examined whether memory recognition continues to engage the hippocampus and other MTL structures over the long term or whether the memory comes instead to rely entirely on the neocortex in the course of sleep-assisted consolidation. They found that recognition memory was associated with increased MTL activity at both early and late time points, with the surviving memory traces becoming more robust in and around the hippocampus in the weeks after encoding and persisting for more than 200 days. This finding is inconsistent with aspects of the active systems consolidation framework, favoring the idea that the hippocampus remains involved in those memories that retain detailed episodic information, as suggested by multiple trace theory ( 27 ).
The systems consolidation account for the role of sleep in memory consolidation is not the only viable notion of how sleep affects the brain. According to the synaptic homeostasis hypothesis, there is a net, and unsustainable, increase in synaptic strength in brain circuits that accumulates during exposure to the events of the day ( 12 ). Sleep promotes a general downscaling of synaptic weights as an antidote. Synaptic downscaling, in this view, avoids saturation of synaptic connections and keeps the high energy costs of synaptic activity under control. Because sleep-dependent downscaling is thought to be selective and to afford relative protection to synapses recently engaged in new learning, sleep-dependent downscaling could also promote memory consolidation by increasing signal to noise in key brain regions. To test this hypothesis, Vanasse et al. ( 26 ) examined whether the relative enhancement of some memory traces is linked to the concomitant forgetting of others. They found that maximal forgetting of learned material (images) overall was positively correlated with stronger brain activation generated by surviving memory traces, which they argue might reflect a reduction of noise in the system. Given that only postsleep recognition memory displayed this noise removal effect, the authors suggest that sleep may have renormalized synaptic weights, which in turn, produced the increased activation of surviving memory traces. While an increased memory signal alone would support the active systems model of consolidation discussed above, the observed correlation with forgetting supports the synaptic homeostasis hypothesis, whereby synaptic downscaling results in the selective preservation of some (previously active and/or especially relevant) memories but not others.
This idea may be related to the selective memory effects observed by Denis et al. ( 13 ) noted above, namely that the emotional elements of one’s experience are selectively retained postsleep, even while neutral elements deteriorate. Of course, such emotional selectivity in memory could reflect active consolidation of important memories only, which underscores the idea that active systems and synaptic downscaling accounts may complement each other.
Using a rodent model, Pedrosa et al. ( 28 ) also focus on hippocampal–neocortical interactions during sleep-based memory consolidation. In the rodent literature, numerous studies have demonstrated an association between “replay” of memories in the hippocampus and several cortical areas, including the prefrontal, visual, retrosplenial, and entorhinal cortices (e.g., 29 , 30 , and 31 ). Exactly how hippocampal activity relates to this broader, cortex-wide activity is not well understood. Pedrosa et al. ( 28 ) used voltage imaging, electrocorticography, and laminarly resolved hippocampal potentials in mice to provide a wide-scale picture of spontaneous cortical activity during sleep and to examine how this activity organizes itself into functional networks. Their data-driven procedure revealed spontaneous neocortical activation signals spanning various spatial scales, which were organized in a small number of functional networks (retrosplenial cortex and medial cortical bank of the cortex, somatosensory cortex, and lateral cortex). The authors then analyzed the hippocampal CA1 layer–resolved local field potential correlates of spontaneous waves involving these three cortical networks. They found that a particular form of brain oscillatory activity, “slow gamma” (20 to 50 hertz), was strongly correlated with the retrosplenial network in particular. This, they claim, argues for a role for slow gamma in memory processing, such that spontaneous activity in the cortex acts as a “cue” for such processing, indicating that interactions between the neocortex and hippocampus are bidirectional. Importantly, the retrosplenial network most involved in this spontaneous dialogue strongly overlaps with the DMN, pointing to a potentially dynamic interchange that may help us understand the involvement of the DMN in memory ( 22 ).
Aleman-Zapata et al. ( 32 ) explored the connection between hippocampal ripples and high-frequency oscillations in the cortex during sleep-dependent consolidation after one-trial spatial learning in rats. With this hippocampal ripple–dependent task, they report cortical oscillations of two high frequencies, with each high frequency involving a distinct neural network—a prefrontal–parietal network for faster oscillations and a hippocampal–parietal network for slower oscillations. Disrupting hippocampal ripples reduced learning and diminished parietal high-frequency oscillations, suggesting that when learning is interrupted, there is less of a need for information in cortex to be consolidated.
The issue of spontaneous hippocampal–neocortical dialogue is further explored in a computational model by Singh et al. ( 33 ), who raise the question of how these brain systems are able to interact and accomplish useful learning and representational sculpting during periods with virtually no environmental input. Their proof-of-concept neural network model shows that when new information is acquired, the hippocampus can facilitate stabilization and integration during sleep by replaying the newly learned neural representations, providing the opportunity for the cortex to integrate and dissect common features of the material being learned. Their model is able to account for the fact that sleep is particularly supportive of new learning about aspects of experience that share features in categorically meaningful ways. Their model also provides insight into the dual roles of consecutive, alternating NREM and REM epochs, wherein NREM facilitates the stabilization of the newly learned representations and REM sleep reduces potential interference between old and new neocortical representations that share overlapping features. Their contribution provides insight into how consolidation mechanisms are initiated and maintained without external influence. However, as already noted, Guthrie et al. ( 15 ) demonstrated that sleep stages in the neocortex and hippocampus can diverge, so it will be important to see if and how future versions of this model could account for divergent sleep stages influencing hippocampal–cortical communication during consolidation.
Although most prior studies have linked the mechanisms of sleep and memory processing indirectly, more recent work has used specific facets of sleep to experimentally and directly alter the fate of memories. One means of doing so involves the use of a procedure called targeted memory reactivation (TMR). A seminal study in humans examined the effect of presenting olfactory cues during sleep that had been part of memory associations learned during the day. In this study, these olfactory cues, which did not wake the participants, improved memory performance ( 34 , 35 ). A growing body of literature has established that presenting olfactory or auditory reminder cues during sleep, especially during slow-wave sleep, can meaningfully boost memory consolidation ( 36 ). Other effects of TMR have also been demonstrated, including studies that have tried to weaken, instead of strengthen, certain memory traces ( 37 , 38 ).
Ngo and Staresina ( 39 ) take the novel step of pairing TMR with experimental augmentation of slow-wave sleep, another method of experimentally enhancing memory consolidation ( 40 ). By combining the two approaches, these authors show that delivering TMR cues during the depolarizing up states of slow oscillations both triggered strong reactivation of memory representations and led to enhanced memory consolidation as measured by improvement in performance. Ngo and Staresina ( 39 ) speculate that the relatively late increase in reinstatement they observe in the scalp electroencephalography (EEG; at about one second) might reflect a slower hippocampal contribution to memory reactivation. However, they note that intracranial EEG recordings would be necessary to test this conjecture.
Creery et al. ( 41 ) do just that by testing five patients with depth electrodes implanted in or near the hippocampus to determine the feasibility of surgery to relieve their epilepsy. While patients slept in the hospital, EEG responses to sounds (half of which had previously been associated with spatial memories) were recorded. These sounds elicited oscillatory intracranial EEG activity increases in the theta, sigma, and gamma EEG bands, with gamma responses in particular predicting the degree of improvement in memory after sleep. Similar to the results presented by Pedrosa et al. ( 28 ), this study provides additional evidence that gamma oscillations might be especially important for sleep-based memory-processing effects.
Sleep and Dreaming
One of the most fascinating aspects of sleep is the occurrence of dreams. Simor et al. ( 42 ) provide a novel multicomponent neurocognitive framework for the onset and maintenance of what is known as lucid dreaming. They extend and build upon prior theories of dreaming suggesting that lucid dreaming is induced when top-down models of the self clash with bottom-up interoceptive pathways, generating prediction errors. They argue that when prediction error is high between current dream content and interoceptive input signaling, the body is in a state of muscle atonia, and lucid dreaming can arise. In nonlucid dreamers, the same mismatch and resultant prediction error might result in sensory input being incorporated into the dream or in an arousal from sleep. Further, top-down attentional control governs prediction error, attenuating the contrast between dream content (i.e., flying) and the true state of the body and motor system, determining whether arousal, dream inclusion, or a lucid dream state results.
This Special Feature provides a current, although necessarily selective, snapshot of the broad and exciting landscape of the field of sleep research and a sense of where the field is moving. The papers included here highlight the dynamic nature of sleep, tying in the role of the autonomic system and shedding light on the mechanisms influencing the sleep–memory connection and how it changes across the life span. They also address aspects of one of the most fascinating aspects of sleep—the nature of dreaming and in particular, the phenomenon known as lucid dreaming. While a full understanding of the range of functions of sleep remains elusive, this set of papers highlights aspects of sleep and sleep research that will eventually provide the detailed mechanistic picture such understanding requires.
The authors declare no competing interest.
Advertisement
Supported by
Nobel Physics Prize Awarded for Pioneering A.I. Research by 2 Scientists
With work on machine learning that uses artificial neural networks, John J. Hopfield and Geoffrey E. Hinton “showed a completely new way for us to use computers,” the committee said.
- Share full article
Two Scientists Awarded 2024 Nobel Prize in Physics
John j. hopfield and geoffrey e. hinton were awarded the prize “for foundational discoveries and inventions that enable machine learning with artificial neural networks.”.
The Royal Swedish Academy of Sciences has today decided to award the 2024 Nobel Prize in Physics to John Hopfield, Princeton University, U.S.A., and Geoffrey Hinton, University of Toronto, Canada, for foundational discoveries and inventions that enable machine learning with artificial neural networks.

By Derrick Bryson Taylor Cade Metz and Katrina Miller
John J. Hopfield and Geoffrey E. Hinton received the Nobel Prize in Physics on Tuesday for discoveries that helped computers learn more in the way the human brain does, providing the building blocks for developments in artificial intelligence.
The award is an acknowledgment of A.I.’s growing significance in the way people live and work. With their ability to make sense of vast amounts of data, artificial neural networks already have a major role in scientific research, the Nobel committee said, including in physics, where it is used to design new materials, crunch large amounts of data from particle accelerators and help survey the universe.
The machine learning breakthroughs of Dr. Hopfield and Dr. Hinton “have showed a completely new way for us to use computers to aid and to guide us to tackle many of the challenges our society face,” the Nobel committee said .
Neural networks — systems that learn skills by analyzing data and are named after the web of neurons in the human brain — are a part of everyday internet services, including search engines like Google, talking digital assistants like Apple’s Siri and chatbots like OpenAI ChatGPT. These services are rooted in mathematics and computer science, not physics.
But research by Dr. Hopfield and Dr. Hinton in the late 1970s and early 1980s helped influence the development of the digital neural networks that have become part of the fabric of the modern internet.
“If there was a Nobel Prize for computer science, our work would clearly be more appropriate for that,” Dr. Hinton, a recipient of the 2018 Turing Award who has been called the “godfather of A.I.,” said in a phone interview with The New York Times. “But there isn’t one.”
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

COMMENTS
The human brain is the command centre for the nervous system and enables thoughts, memory, movement, and emotions by a complex function that is the highest product of biological evolution. Maintaining a healthy brain during one's life is the uppermost goal in pursuing health and longevity. As the population ages, the burden of neurological ...
Brain articles from across Nature Portfolio. The brain is the part of the central nervous system that is contained within the skull. It is responsible for executive and cognitive functions and ...
Functional brain activity is often measured during a single scanning session which neglects behavioral, physiological and lifestyle effects on brain activity. This study presents functional neuroimaging data from 30 neuroimaging session across 15 weeks from one participant and reveals relationships between human daily behavior and brain function under different stimuli.
Author Summary By analogy with the road network, the human brain is defined both by its anatomy (the 'roads'), that is, the way neurons are shaped, clustered together and connected to each others and its dynamics (the 'traffic'): electrical and chemical signals of various types, shapes and strength constantly propagate through the brain to support its sensorimotor and cognitive ...
A brain circuit that cements the memory of socially learnt food preferences. How an experience results in a long-term memory has remained unknown. A circuit involving the posteromedial nucleus of ...
Using multimodal brain imaging and organ-specific physiological markers from more than 18,000 adult participants of the UK Biobank database, this study reveals integrated pathways that explain the ...
David Z Wang and colleagues look at the latest advances in brain research and how they might affect treatment of brain disorders The world has come a long way in solving the mystery of the brain, understanding its fundamental role in human consciousness and discovering methods to treat its disorders. In The Sacred Disease in ~430 BC, Hippocrates wrote that the brain served to house the ...
However, deep-brain stimulation as a treatment option for Parkinson disease, depression, or addiction is a different story. 60-62 Additionally, research on so-called brain/machine interfaces (BMIs) has shown that with regard to motor functions and the assimilation of artificial tools, eg, robotic/avatar extremities, incorporation in the ...
Brain Research is dedicated to publishing the highest quality and greatest impact articles within the ever-evolving field of Neuroscience. We recognize how technology has changed the way scientific breakthroughs are communicated and Brain Research is committed to serving as a dynamic journal …. Between 1981 and 1988 Brain Research shared its ...
Our brain is composed of 86 billion neurons and a similar number of non-neuronal cells. The National Institute of Health's Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative - Cell Census Network (BICCN), which was first launched in 2017, is a consortium of centers distributed in the United States and Europe that work together toward the goal of characterizing ...
1. Introduction. How the human brain works is still an open question, as is its implication with brain architecture: the non-trivial structure-function relationship. The development of neuroimaging techniques, the improvement on their processing methods and the unfolding of computational neuroscience field have driven brain research focused ...
May 9, 20243 min read. Researchers publish largest-ever dataset of neural connections. A cubic millimeter of brain tissue may not sound like much. But considering that that tiny square contains 57,000 cells, 230 millimeters of blood vessels, and 150 million synapses, all amounting to 1,400 terabytes of data, Harvard and Google researchers have ...
Yongjun Wang and colleagues discuss the definition of brain health and the opportunities and challenges of future research The human brain is the command centre for the nervous system and enables thoughts, memory, movement, and emotions by a complex function that is the highest product of biological evolution. Maintaining a healthy brain during one's life is the uppermost goal in pursuing ...
• An article about brain trauma data from the U.S. Department of Veterans Affairs revealing that women have a more difficult recovery from severe brain trauma than men do. ... It was a tough sell to other neurosurgeons, but research by Gregory Albers, MD, and a team of researchers eventually succeeded in extending the window for effective ...
In addition to describing the FlyWire brain resource, this Article also presents analyses that illustrate how the data products can be used. Additional whole-brain network analyses are provided by ...
As a result, research on the teenage brain is finally starting to catch up with studies of other age groups, complete with the level of detail it deserves. "The shift from childhood to adulthood is not a linear one. Adolescence is a time of wonderfully dynamic change in the brain," said BJ Casey, ...
Source memory improves substantially during childhood. This improvement is thought to be closely related to hippocampal maturation. As previous studies have mainly used cross-sectional designs to assess relations between source memory and hippocampal function, it remains unknown whether changes in the brain precede improvements in memory or vice versa. To address this gap, the current study ...
Research on relationships, new treatments for mental health conditions, and more. ... 2024 — Deep brain stimulation may provide immediate improvement in arm and hand strength and function ...
There are clear indications that PA also has important effects on human brain health at any age and have been included, for example, in the Physical Activity Guidelines for Americans, issued by the U.S. Department of Health and Human Services (HHS) in 2018 [17, 18, 19]. Interestingly, in these guidelines, four classes of age, with different PA ...
Neuroscience News Home. Neuroscience News is an independent open access science magazine. Since 2001, we have featured neuroscience research news from labs, universities, hospitals and news departments around the world. Topics include brain research, AI, psychology, neuroscience, mental health and neurotech.
One leading brain specialist independent of the new research described the breakthrough as a "huge leap" in our understanding of our own brains. One of the research leaders said it would shed new ...
Clearing waste from the brain. Scientists believe this network of pathways effectively flushes the brain of metabolic wastes generated by its energy-intensive work. Wastes include proteins such as amyloid and tau, which have been shown to form clumps and tangles in brain images of patients with Alzheimer's disease.
The brain. This supremely complex organ is slowly giving up its valuable secrets. In the hand, the human brain is a jelly-like mass, easily deformed by touch. However, its unassuming appearance ...
The human brain is a 3-pound (1.4-kilogram) mass of jelly-like fats and tissues—yet it's the most complex of all known living structures. The brain is extremely sensitive and delicate, and so it ...
Take this 2015 study on sex differences in the human brain. It reported on 34,716 different patterns of functional brain connectivity, and found statistical differences between females and males ...
Moreover, insights from brain research will increasingly influence learning and education and have an impact on our society. To stay ahead of emerging ethical, societal and legal issues, and to strengthen the societal benefit and acceptability of its findings, EBRAINS need structures and strategies for engaging in dialogue with communities on ...
This work is expected to help scientists to identify suitable animal models of human brain conditions — such as Parkinson's disease, motor neuron disease and Alzheimer's disease — that ...
Patients were divided into two groups: the group C (N = 7) with caudal brain stem lesions and the group R (N = 7) with rostral brain stem lesions as defined by the midpons level. The patient group was compared to a control group of 20 healthy subjects.
Keywords: sleep, memory, function, cognitive neuroscience. One link between sleep and the brain concerns the processes by which newly acquired information is stored. The notion that sleep benefits memory dates back to Ebbinghaus (6), who pioneered the experimental study of memory by demonstrating the effect of time on forgetting.
With work on machine learning that uses artificial neural networks, John J. Hopfield and Geoffrey E. Hinton "showed a completely new way for us to use computers," the committee said.