The silver mirror reaction
The scientific description of an amazing experiment
The experiment that has a beautiful effect of a mirror surface forming on glass looks very impressive. Carrying out of this reaction requires experience and patience. In our article, you can read about the necessary reagents , how to prepare the equipment, and to find out the chemical equations of reactions.
The essence of the silver mirror reaction is the formation of metallic silver layer as a result of an oxidation-reduction reaction, by the interaction of an ammonia solution of silver oxide in the presence of aldehydes.

To make a durable silver layer, you will need:
- 100ml glass flask;
- 2,5-4% ammonia solution;
- silver nitrate 2%;
- formaldehyde solution (40%).
Instead of the ammonia and silver nitrate solutions, you can also use Tollens’ reagent – an ammonia solution of silver oxide. Add 1 gram of silver nitrate to 10 drops of water (if the liquid is supposed to be stored for a long time, keep it in a dark place or in a dark glass bottle). Before the experiment, mix the solution (around 3 ml) in the proportion of 1/1 with a 10% sodium hydroxide solution. There may be a precipitate of silver, which you can dilute by slowly addition of ammonia solution. The reaction occurs at room temperature. For the successful result, the walls of the glass flask must be perfectly clean and smooth. If there are tiny particles of dirt on the walls, the obtained precipitate will appear as a crumbly layer of a black or dark grey color.
To clean the flask, use different types of alkaline solutions. First, use a caustic soda solution, and then rinse the flask with distilled water. Rinse the flask with the cleanser many times.

What can the silver mirror reaction show
This interesting chemical reaction not only allows us to examine certain states of a substance, but can also give an exact definition of aldehydes. This reaction answers the question: is there an aldehyde group in the solution or not?
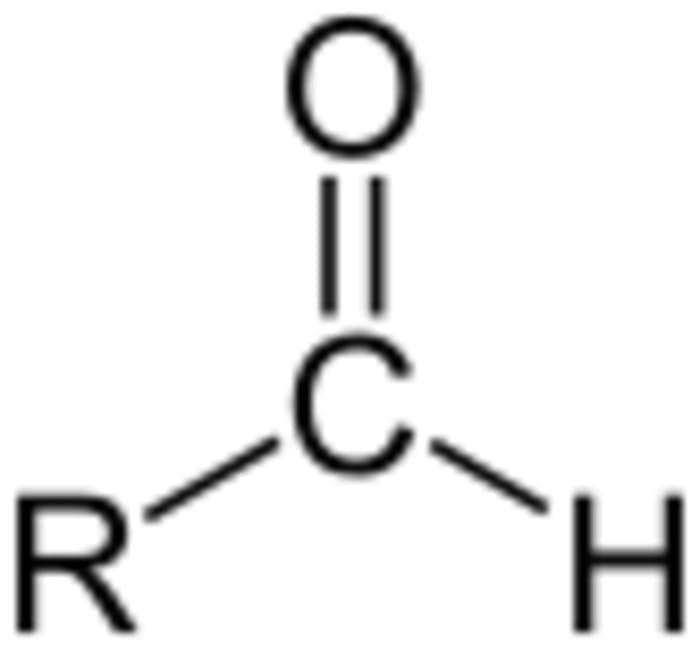
For example, due to this process, we can find out what component the solution contains: glucose or fructose. Glucose gives a positive result, a “silver mirror” is formed, while fructose contains a ketone group and cannot form a silver precipitate. To make an analysis, you should add a 10% glucose solution instead of a formaldehyde one. Let’s have a look at the equation to see why and how the dissolved silver turns into a solid precipitate:
2[Ag(NH₃)₂]OH + 3H₂O + C₆H₁₂O₆ (glucose) = 2Ag↓+ 4NH₃∙H₂O + C₆H₁₂O₇ (gluconic acid forms).


Dozens of experiments you can do at home
One of the most exciting and ambitious home-chemistry educational projects The Royal Society of Chemistry

IMAGES
VIDEO