- Experiments

Cupric sulfate
Grow copper (II) sulfate crystals

- Copper(II) sulfate
Put protective gloves on.
Conduct the experiment on the tray.
Observe safety precautions when working with boiling water.
- Do not allow chemicals to come into contact with the eyes or mouth.
- Keep young children, animals and those not wearing eye protection away from the experimental area.
- Store this experimental set out of reach of children under 12 years of age.
- Clean all equipment after use.
- Make sure that all containers are fully closed and properly stored after use.
- Ensure that all empty containers are disposed of properly.
- Do not use any equipment which has not been supplied with the set or recommended in the instructions for use.
- Do not replace foodstuffs in original container. Dispose of immediately.
- In case of eye contact: Wash out eye with plenty of water, holding eye open if necessary. Seek immediate medical advice.
- If swallowed: Wash out mouth with water, drink some fresh water. Do not induce vomiting. Seek immediate medical advice.
- In case of inhalation: Remove person to fresh air.
- In case of skin contact and burns: Wash affected area with plenty of water for at least 10 minutes.
- In case of doubt, seek medical advice without delay. Take the chemical and its container with you.
- In case of injury always seek medical advice.
- The incorrect use of chemicals can cause injury and damage to health. Only carry out those experiments which are listed in the instructions.
- This experimental set is for use only by children over 12 years.
- Because children’s abilities vary so much, even within age groups, supervising adults should exercise discretion as to which experiments are suitable and safe for them. The instructions should enable supervisors to assess any experiment to establish its suitability for a particular child.
- The supervising adult should discuss the warnings and safety information with the child or children before commencing the experiments. Particular attention should be paid to the safe handling of acids, alkalis and flammable liquids.
- The area surrounding the experiment should be kept clear of any obstructions and away from the storage of food. It should be well lit and ventilated and close to a water supply. A solid table with a heat resistant top should be provided
- Substances in non-reclosable packaging should be used up (completely) during the course of one experiment, i.e. after opening the package.
FAQ and troubleshooting
I can’t manage to grow the crystal on the wire. what to do.
There are two scenarios possible. In the first one, crystals form on the bottom of the test tube instead of growing on the wire. If so, carefully cut the wire to expose a clean copper surface from the end you stick into the test tube. Repeat the experiment starting from the step 3 of the instruction.
In the second scenario, crystals do grow on the wire, but there are too many of them, and they are small and merging with each other. Well, even a bunch of small crystals looks marvelous, doesn’t it? Still, in order to grow one big crystal, you have to repeat the experiment. First, carefully shake off the crystals from the wire back into the test tube and then follow the instructions starting from the step 3. Note that water in the step 10 must be hot! Remember that growing a nice big crystal requires the whole setup to be cooled gradually.
In any scenario, remember that crystals growing is an art. Don’t get frustrated if you couldn’t manage it from the first or even the second attempt. Have some patience, and you’ll grow your best crystal at last!
Step-by-step instructions
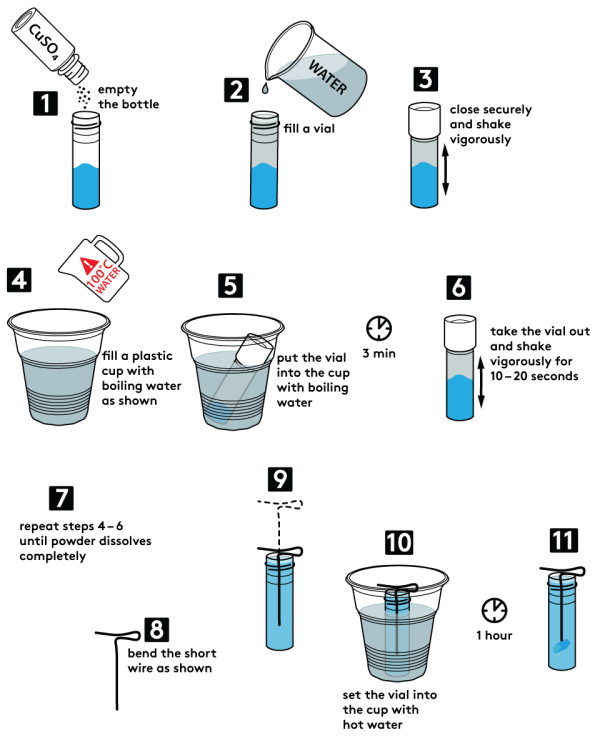
- Pour all the copper sulfate bottle contents (5 g) into an empty plastic vial.
- Fill the vial with water up to the top.
- Close the vial tightly and shake it a few times.
- Pour boiling water into a plastic cup, as shown. Use caution when working with boiling water!
- Place the vial with copper sulfate into the cup with boiling water. Wait for 3 minutes.
- Carefully draw the vial from the cup. Shake it thoroughly for 10–20 seconds.
- Repeat steps 5 and 6 until all copper sulfate is dissolved.
- Form a short piece of copper wire into a figure, as shown. Make sure that it can be securely mounted inside the vial.
- Place the copper wire into the vial with copper sulfate solution.
- Place the vial back into the cup with hot water. Wait for one hour.
- A crystal grown on the copper wire! You may leave the wire in the vial for another 1–2 hours; the crystal will grow even bigger.
Expected result
A blue transparent crystal of cupric sulfate CuSO 4 *5H 2 O grows on a copper wire.
Dispose of the experiment residues along with regular household trash.
Scientific description
Why do crystals grow.
Copper sulphate belongs to those substances that dissolve in water better upon heating. And vice versa, their solubility decreases upon cooling, which in our case leads to precipitation of copper sulfate in form of beautiful blue crystalline hydrate CuSO 4 ·5H 2 O. Due to the fact that the solution is cooled down slowly, the crystals grow gradually and only on a wire.
Why does the copper sulfate solution tend to form crystals but not a fine powder upon cooling? Crystals are very much different from amorphous solids (such as carbon black or glass): particles they consist of are arranged among themselves in a strict geometrical order inside a solid. Though, such clear complementarity is often unfavorable to the nature. And still, such an arrangement of particles within a solid “feels” very comfortable to them. This means that each atom is strongly connected with its surroundings, and that all positive charges closely interact with all negative charges.
For example, copper atom prefers to be surrounded by six oxygen atoms. Such an environment is observed in crystals of hydrate:
Why do crystals grow on a copper wire?
Like attracts like. There is always a thin layer of copper oxide (CuO) on a wire surface. Copper within this layer is in the same oxidation state Cu 2+ as in copper sulfate. In addition, the surface of copper wire is rough by itself, and there are many small bumps in it. When copper sulfate feels “too tight” in the solution, it starts to crystallize on the wire because it has irregularities and also contains copper.
Typically, substance crystallization begins with so-called crystallization nuclei. Each crystallization nucleus is a group of particles that resembles future crystals by composition. Crystal growth starts from such nuclei, similar to a plant growing from a seed in the ground. If there were no wire in the bottle, the growth would start from any point where a seed crystal first appeared. However, the surface of a copper wire is so constructed that it promotes the formation of copper sulfate crystallization nuclei directly on it. This feature, along with slow cooling of the solution, provides for the crystals grow only on the wire surface.

Why should the bottle be kept in hot water during crystal growth?
In the other experiments of this set, cooling of a crystallizing compound solution occurs at room temperature. However, in this experiment, crystallization process is slowed down by letting the solution to cool gradually in hot water. It turns out, if a bottle with hot saturated solution of copper sulfate is immersed immediately in cold water (or is simply left in air), then blue crystals begin to grow actively not only on a wire, but also at the bottom of the bottle. In that case, crystals grow overlapping each other, resulting in quite a nice, but rather shapeless forest. But we want the crystals to grow neatly, and the experiment to turn out spectacular. Therefore, in order to grow the crystals solely on the wire, we need to arrange for a very slow cooling. The latter is achieved with the thoroughly selected experiment conditions.
Ideally, we want to ensure that the crystals grow not only on one particular spot, but also in a certain way. The luckiest experimenters have been able to achieve the most interesting results: almost all the copper sulfate has been grown in the form of a so-called single crystal. It is a beautiful polyhedron, glossy and transparent on all sides, without any extra features or irregularities inside.
Most often, though, a few crystals fuse with each other irregularly, and some of their parts are clearly rotated relative to the other. Inside a solid, may be seen heterogeneity, cracks, and sometimes even small crystals grown into a large one. These “anomalies” are always caused by deviations from the ideal experiment conditions. Thus, the ideal conditions imply very slow changes of all factors influencing a crystal growth process. They include solution temperature, atmospheric pressure, and absence of any vibration or other disturbances. So, oddly enough, the “set and forget” approach is really ideal for the crystals growth.
Big crystal
Small blue crystals of copper sulphate, without doubt, delight the eye. But what about growing a very big crystal? However, that is not so easy.
As a container, use a plastic cup (that way you can heat it just like the glass of tartaric acid or sugar in the other experiments of the kit) or a glass beaker. In the first case, you will need about 30 grams of copper sulfate CuSO 4 *5H 2 O. You can find it in a store where fertilizers are sold, or in a hardware store. If you decided to grow a very large crystal and to do it in a beaker, prepare in advance 60-70 grams of copper sulfate.
Fully dissolve the copper sulphate in hot water. Thoroughly mix the solution until there are no crystallites remaining. Use a piece of copper wire, thread or a splinter as the "support" of the crystal.
Now please be patient! A large crystal can grow for several days!
Crystallization in the refrigerator
How does the environmental temperature affect the rate and the result of crystallization? You can research that! Repeat the experiment, however prepare two test tubes with copper sulfate solution at the same time. In each of them you will need to add 5 grams CuSO 4 *5H 2 O, so use the solution and the crystals from the main experiment.
Stop after the 9th step in the instructions. Now, put one of the test tubes in a cup with hot water, as indicated in step 10, and put the second in the refrigerator (the temperature inside is about 4 C o ).
Wait for 1-2 hours. Compare the results. Where did the crystal grow bigger? Where are there more of them and why?
Crystals of NaCl
Try to grow a crystal of the most common table salt - sodium chloride NaCl.
Dissolve 39 grams of salt in 100 ml of boiling water. Thoroughly mix the solution until there are no crystallites remaining. As the "support" for the crystal is best to use a thread wound on a splinter - lower its end into the solution. Tie a couple of knots on the end of the string - it may help.
Now all you have to do is wait! Make sure the glass is in a place where no one will shake or drop it over.
That’s interesting!
Why grow crystals.
Many synthetic chemists discover and use different methods of single crystals growth in their work. So, why is it attractive and beneficial to professional chemists?
Besides an aesthetic effect (“I have synthesized a substance, and it forms beautiful crystals!”), there is a compelling need to asseamble molecules of new or unknown substances into perfectly ordered single crystals. Normally, after synthesizing a new compound, a chemist has to clarify (confirm) its molecular structure. Until he or she does that, no one in the world scientific community accepts his or her discovery.
There are numerous indirect methods to examine a substance molecular structure. For instance, chemists may expose a substance to visible or infrared light, strong magnetic field or another physical load, looking for hints about the order its atoms are arranged within molecules.
Among these methods, the most reliable and common approach to defining a new compound structure is a so-called X-ray crystallography analysis. It allows researchers to take a “snapshot” of a new substance lattice. This information, in turn, can immediately answer all questions about its molecular structure. Despite its effectiveness, though, this method has a very significant drawback: a substance must be analyzed in form of a single crystal.
It should be understood that each molecule, even if we are talking about very large molecules of polymers or proteins, is very, very small, which can only be detected with the help of special equipment and under strict conditions. Thus, unveiling the structure of a single molecule requires additional finesse. However, even a milligram of any substance contains a huge amount of identical molecules. If you assume that all the molecules respond equally to the same external exposure, and then sum up all these responses, then detection of that bulk signal becomes much easier.
As mentioned earlier, single crystals are unique in a way that their constituent “blocks” are in a strictly defined repetitive order. It allows for summing up the molecules responses to a certain effect, as they are all organized in space in the same way. X-ray analysis method assumes that substance molecules are responsive to X-ray irradiation. After these rays reach the substance molecules, they change their direction in a specific way, which depends on the arrangement of atoms in a single crystal.
Further, scientists analyze the pattern created by diffracted rays to define where atoms, which caused such a change, are located in the crystal. This knowledge makes it possible to figure out the substance molecular structure. Quite simply, if an atom is not a part of a molecule, then in most cases it will be detected being away from all other atoms in the molecule, at a distance of more than 3.5 angstroms, which is one hundred million times less than 3.5 centimeters.
By a curious coincidence, X-rays are also used to examine internal structures of a human body, as well as of many other living creatures. For example, in case of a bone fracture, X-ray radiograms (or just X-rays) of damaged body parts are taken, which lets a doctor know where exactly the fracture is located and how to treat it efficiently.

Dozens of experiments you can do at home
One of the most exciting and ambitious home-chemistry educational projects The Royal Society of Chemistry
JavaScript is required...
Please enable Javascript in order to use PubChem website.

IMAGES
VIDEO
COMMENTS
Apparatus1. Eye protection 2. Test tubes x2 3. Delivery tube (right-angled) 4. Beaker, 250 cm3 5. Bunsen burner 6. Clamp and stand…
In the other experiments of this set, cooling of a crystallizing compound solution occurs at room temperature. However, in this experiment, crystallization process is slowed down by letting the solution to cool gradually in hot water.
In this experiment students will measure the mass of hydrated copper(II) sulfate before and after heating and use mole calculations to find the formula.
Try this class practical or demonstration to illustrate the displacement of copper from copper (II) sulfate using aluminium foil. In this experiment, students add aluminium cooking foil to copper (II) sulfate solution and observe no reaction.
In this experiment, you will prepare copper metal from the reaction of aluminum metal with a solution of copper(II) sulfate (cupric sulfate). From the amounts of the reactants, you will …
To prepare a pure, dry sample of hydrated copper(II) sulfate crystals. Materials. 1.0 mol / dm 3 dilute sulfuric acid; Copper(II) oxide; Spatula & glass rod; Measuring cylinder & 100 cm 3 beaker; Bunsen burner; Tripod, …
Cupric sulfate is a salt created by treating cupric oxide with sulfuric acid. This forms as large, bright blue crystals containing five molecules of water (CuSO4∙5H2O) and is also known as blue vitriol. The anhydrous salt is …
Here is how you make copper sulfate yourself, using a battery, copper wire, and dilute sulfuric acid. Materials for Making Copper Sulfate. The easiest and safest method of making copper sulfate uses electrochemistry. …