PH Levels Of Catalase

Catalase is an enzyme, a protein that catalyzes or accelerates chemical reactions. In the human body, catalase breaks down hydrogen peroxide in the liver, which is important for certain reactions in cells but can also damage DNA. Catalase prevents damage by accelerating the breakdown of hydrogen peroxide into oxygen and water. If you pour hydrogen peroxide on a cut, you may notice bubbling. The bubbles are oxygen gas caused by a reaction with catalase.

TL;DR (Too Long; Didn't Read)
Catalase is an enzyme, a large protein that speeds up the rate of a chemical reaction. The optimum pH level of catalase is between pH 7 and pH 11. At a pH level lower or higher than this range, the catalase stops working.
Enzyme Activity
To work well (or at all) an enzyme needs a certain environment or condition. As temperature increases, the rate of enzyme activity also increases. As temperature increases toward its optimum point of 37 degrees Celsius (98.6 F), hydrogen bonds relax and make it easier for the hydrogen peroxide molecules to bind to the catalase. The part of the enzyme where this reaction takes place is called the active site. A temperature that is higher or lower than this optimum point changes the shape of the active site and stops the enzyme from working. This process is called denaturation.
Catalase pH Levels
Enzyme pH levels also change the shape of the active site and affect the rate of enzyme activity. Each enzyme has its own optimal range of pH in which it works most effectively. In humans, catalase works only between pH 7 and pH 11. If the pH level is lower than 7 or higher than 11, the enzyme becomes denaturated and loses its structure. The liver sustains a neutral pH of about 7, which creates the best environment for catalase and other enzymes.
Measuring Catalase Activity
You measure catalase activity by adding catalase solution to hydrogen peroxide solution and leaving it for a length of time – one minute, for example. The reaction produces bubbles of oxygen gas, which look like foam. Use a ruler to measure the height the foam reaches in the test tube. The higher the foam in the test tube, the greater the catalase activity. Vary the pH level and temperature of the solution to investigate the effects of pH and temperature on enzyme activity. Hydrogen peroxide is exceptionally corrosive, so handle with care and wear safety goggles throughout the experiment.
- Saylor Academy: Catalase
- Digital Learning for Wales: Experiment: The Effect of pH on Catalase Activity
- Scientific American: The Liver: Helping Enzymes Help You!
Cite This Article
Gillespie, Claire. "PH Levels Of Catalase" sciencing.com , https://www.sciencing.com/ph-levels-catalase-6826245/. 21 May 2018.
Gillespie, Claire. (2018, May 21). PH Levels Of Catalase. sciencing.com . Retrieved from https://www.sciencing.com/ph-levels-catalase-6826245/
Gillespie, Claire. PH Levels Of Catalase last modified August 30, 2022. https://www.sciencing.com/ph-levels-catalase-6826245/
Recommended
Investigation: Enzymes

Enzyme Lab Teacher's Guide
Measure the effects of changes in temperature, pH, and enzyme concentration on reaction rates of an enzyme
Explain how environmental factors affect the rate of enzyme-catalyzed reactions.
INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances.
Enzymes are proteins that speed up the rate of reactions that would otherwise happen more slowly. The enzyme is not altered by the reaction. You have hundreds of different enzymes in each of your cells.
Each of these enzymes is responsible for one particular reaction that occurs in the cell. In this lab, you will study an enzyme that is found in the cells of many living tissues. The name of the enzyme is catalase; it speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances--water and oxygen. Light can also break down H 2 O 2 which is why the chemical is sold in dark containers.
The reaction is: 2H 2 O 2 → 2H 2 O + O 2
This reaction is important to cells because hydrogen peroxide is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, they would be poisoned and die. In this lab, you will study the catalase found in liver cells. You will be using chicken or beef liver. It might seem strange to use dead cells to study the function of enzymes. This is possible because when a cell dies, the enzymes remain intact and active for several weeks, as long as the tissue is kept refrigerated.
MATERIALS: 6 Test tubes / Test tube holders 3% Hydrogen peroxide Ice / Hot water Straight-edged razor blade Scissors and Forceps Measuring Pipettes Stirring rod
Fresh liver, Apple, and Potato, Yeast Vinegar / Baking Soda HCL and NaOH pH paper (optional)
PART A - Observe Normal Catalase Reaction
1. Place about 2 ml of the 3% hydrogen peroxide solution into a clean test tube.
2. Using forceps and scissors cut a small piece of liver and add it to the test tube. Push it into the hydrogen peroxide with a stirring rod. Observe the bubbles. What gas is being released? (consider the equation above) _____
Throughout this investigation you will estimate the rate of the reaction (how rapidly the solution bubbles) on a scale of 0-5 (0=no reaction, 1=slow, ..... 5= very fast). Assume that the reaction in step 2 proceeded at a rate of "4"
Recall that a reaction that absorbs heat is endothermic; a reaction that gives off heat is exothermic. Feel the temperature of the test tube with your hand.
Has it gotten warmer or colder? Is the reaction endothermic or exothermic?
3. Pour off the liquid into a second test tube. Assuming the reaction is complete, what is this liquid composed of?
What do you think would happen if you added more liver to this liquid?
Test this and record the reaction rate. Reaction Rate:
4. Add another 2ml of hydrogen peroxide to the liver remaining in the first test tube. What is the reaction rate?
Synthesis -- Answer the question: Is catalase reusable?
REASONING .
Part B - What Tissues Contain Catalase?
You will now test for the presence of catalase in tissues other than liver. Place 2 ml of hydrogen peroxide in each of 3 clean test tubes and then add each of the three test substances to the tubes. As you add each test substance, record the reaction rate (0-5) for each tube.

Synthesis -- Do all living tissues contain catalase? Claim:
Evidence: Reasoning:
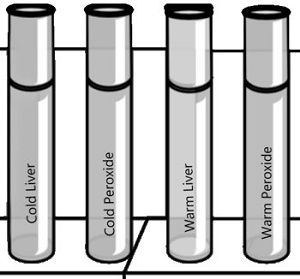
PART C - What is the Effect of Temperature on Catalase Activity?
1. Put a piece of liver into the bottom of a clean test tube and cover it with a small amount of water. Place this test tube in a boiling water bath for 5 minutes.
2. Remove the test tube from the hot water bath, allow it to air cool, then pour out the water. Add 2 ml of hydrogen peroxide. CAUTION: Use a test-tube holder for hot test tubes.
What is the reaction rate for the boiled liver and peroxide? __________
3. Put equal quantities of liver into 2 clean test tubes and 1 ml H 2 O 2 into 2 other test tubes. Put one test tube of liver and one of H 2 O 2 into an ice bath. Place the other set in a warm water bath (not boiling).
After 3 minutes, pour each tube of H 2 O 2 into the corresponding tube of liver and observe the reaction
What is the reaction rate for the cold liver/peroxide? _____ What is the reaction rate for the warm liver/peroxide? ____
Synthesis -- How does temperature affect the catalase enzyme?
Claim:
PART D - What is the Effect of pH on Catalase Activity
1. Add 2 ml hydrogen peroxide to 4 clean test tubes, then add:
Tube 1 – add 3 drops of acetic acid (vinegar) pH =_______ Tube 2 – add 3 drops of sodium bicarbonate (base) pH =______ Tube 3 – add 3 drops of water (neutral) pH =_____ Tube 4 -- add 3 drops of 1M NaOH pH = _____
Now add liver to each of the test tubes (try to do it all at about the same time, so you can easily compare)
Rate of Reaction for:
Strong Acid (HCL) ____ Acid _____ Neutral ______ Base_____ Strong base (NAOH) _____
1. How does pH affect the reaction rate of catalase? Propose a way to refine your experiment to find the exact , or OPTIMAL pH and temperature of catalase.
2. The following graph shows reaction rates of various enzymes in the body. Pepsin is found in the stomach, amylase in the saliva, and phosphatase in the liver.
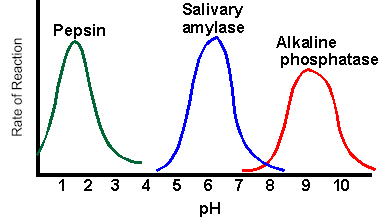
Synthesis: How does pH affect the activity of enzymes?
Claim:

Part E - Design an Experiment
Lactaid is a product designed to help people who cannot digest milk sugar (lactose) because they are missing the enzyme lactase. Many people are lactose-intolerant, a condition that is mainly genetic. Lactase breaks down lactose into two subunits: glucose and galactose.
To test for the presence of monosaccharides and reducing disaccharide sugars in food, the food sample is dissolved in water, and a small amount of Benedict's reagent is added. The solution should progress in the colors of blue (with no glucose present), green, yellow, orange, red, and then brick red when there is a large amount of glucose present. (Google benedict's test to see the way this looks.)
Design an experiment where you would determine how quicly lactaid works to break down milk sugar at different temperatures.. Be specific in your description, use drawings if necessary.
Other Resources on Enzymes
Analyzing Graphics - Enzymes - shows substrates and enzyme interactions and explores competitive inhibition
Observe Catalase Activity in Yeast - create sodium alginate spheres to observe how catalase breaks down hydrogen peroxide
Enzyme Activity Using Toothpickase - simulate the activity of an enzyme by breaking toothpicks.

Catalase Enzyme Lab

Why enzymes are important
Chances are your biochemistry unit is not a student favorite. I often hear “why are we learning chemistry in biology class?” But to really understand how cells work, students need to understand macromolecules. (If you need some biochemistry lesson ideas, check out this blog post ). Luckily, there are fun labs you can do to help students understand enzymes.
Using catalase to test enzyme efficiency
A common enzyme lab for students to measure the impact of temperature and pH on the efficiency of catalase. Catalase is an enzyme is found in almost all living organisms that breaks down hydrogen peroxide (H2O2) into oxygen and water. Many teachers use raw chicken liver or potato as the source of the catalase. I’ve done both and frankly potato is less stinky and is easier to clean up after. Here is the gist of the lab:
- Students will need: potato puree, tweezers, a beaker full of hydrogen peroxide, and a stopwatch.
- Peel a raw potato and cut it into pieces. Place the potato in the blender and add a small amount of water. Puree until smooth. (One large potato should be enough for 1 class period).
- Note: The potato will turn brown relatively quickly as it comes in contact with the air. Don’t worry! This does not impact the results of the experiment.
- Collect the paper discs out of your hole puncher (or hit up the copy center at your school).
- Using tweezers, have students dip a paper disc in the potato puree. Place the paper in the bottom of the beaker of peroxide and start the stopwatch. As the catalase on the paper disc breaks peroxide into oxygen and water, the disc will float. Have students time how long it takes for the paper to rise.
- Concentration: To show students the impact of concentration on enzyme efficiency, simply water down the potato puree. Do a trial with straight potato, a trial with a 3:1 ratio of potato to water, and a trial with 1:1 ratio of potato to water.
- pH: To show students the impact of pH on enzyme efficiency, have them add a few drops of an acid and a base to the potato purees on a spot plate. Vinegar and bleach are great options. Repeat the experiment and have students determine at which pH catalase works best.
- Option 1: Change the temperature of the peroxide. Place a beaker of peroxide in an ice bath, and another in a warm water bath. This option tends to yield the best results.
- Option 2: Change the temperature of the potato puree. This can be done easily by putting some of the puree in the fridge and some in the microwave (or boil it at home ahead of time). This does not always give the best results because the cold potato can warm up pretty quickly, but still works if you don’t have water baths available.
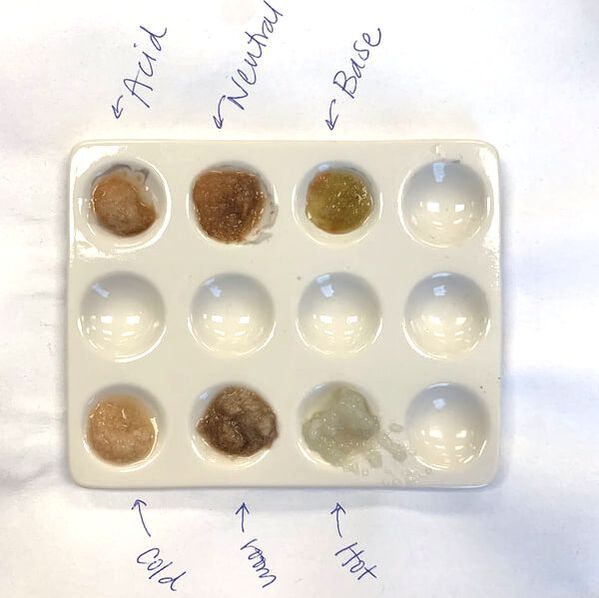

Troubleshooting Tips
After doing this lab for multiple years, here are some additional tips:
- Have students do multiple trials (at least 3) and take the average. Sometimes they get weird data, so this helps with accuracy.
- If you are testing multiple variables, have students get fresh peroxide before starting the new variable. For example, have students collect all the temperature data, get fresh peroxide, and then collect pH data.
- If the paper disc takes more than 1 minute to rise, tell students that the enzyme is denatured and they can stop timing and move on to the next trial.
- When I first started doing this lab I used petri dishes for all the potato purees and it was a lot of clean up. I recently switched to chemistry spot plates (pictured above) and it made clean up so much easier!
If you would like to purchase a lab write-up, you can find it here:

- Read more about: Biochemistry

Hi, I'm Becca!
Search the site, browse by category.
- Back to School
- Biochemistry
- Body Systems
- Classification
- Classroom Decor
- Classroom Management
- Distance Learning
- End of the School Year
- Experiments
- Field Trips
- For NEW Teachers
- Formative Assessment
- Media in the Classroom
- Microscopes
- Photosynthesis & Respiration
- Plate Tectonics
- Sustainability
- Teacher Tips
- Weather and Climate
Get Freebies!
You might also like....

Teaching DNA Structure and Replication just got easier

10 Atmosphere Lessons for High School

Read Aloud Closure Assignments

Want a fun way to practice science vocabulary? Try out seek and finds!

Privacy Overview

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
7 Lab 6. Enzymes – Catalase Function
Lab 6: enzymes – catalase function.
- Practice and apply hypothesis testing.
- Practice experimental design.
- Gain a better understanding of enzymes and some conditions that affect enzyme activity and the rate of an enzyme-catalyzed reaction.
- Understand these terms: enzyme, enzyme activity, active site, substrate, enzyme-substrate complex, product, denature, competitive inhibition, and noncompetitive inhibition.
INTRODUCTION
Enzymes are proteins that increase the rate of chemical reactions. This process is called catalysis . All cells use enzymes for metabolism , the sum of all chemical and physical reactions that a cell uses to break down nutrients to produce energy and to build up important molecules needed for cell function. Enzymes increase the rate of a reaction by lowering activation energy but are not themselves consumed in the reaction. Thus, one enzyme can catalyze repeated rounds of the same reaction.
As with all proteins, an enzyme’s function depends completely on its shape. Enzymes have a very complex three-dimensional structure consisting of one or more polypeptide chains folded to form an active site – a specific area into which the substrate (material to be acted on by the enzyme) will fit. The shape of the enzyme determines which reaction it will catalyze, because only one substrate will fit into its active site.

Changes in temperature, pH, and/or the addition of certain ions or molecules may affect the structure of an enzyme’s active site and thus the ability of the enzyme to catalyze the reaction (the enzyme’s “enzyme activity”). Changing an enzyme’s shape so that it is no longer biologically active is called denaturation . Enzyme inhibitors can occupy the active site (competitive inhibitors) or change the shape of the active site by binding elsewhere (noncompetitive inhibitors). Hence, these factors also affect the rate of the reaction in which the enzyme participates. The rate of an enzymatic reaction can also be affected by the relative concentrations of enzyme and substrate.
Salt concentration: If the salt concentration is very low or zero, the charged amino acid side-chains of the enzyme will stick together. The enzyme will denature and form an inactive precipitate. If, on the other hand, the salt concentration is very high, normal interaction of charged groups will be blocked, new interactions occur, and again the enzyme will precipitate. An intermediate salt concentration such as that of blood (0.9%) or cytoplasm is optimum for most enzymes.
pH: pH is a logarithmic scale that measures the acidity or H+ concentration in a solution. As the pH is lowered, an enzyme will tend to gain H+ ions, and eventually enough side chains will be affected so that the enzyme’s shape is disrupted. Likewise, as the pH is raised, the enzyme will lose H+ ions and eventually lose its active shape. Many enzymes have an optimum in the neutral pH range and are denatured at either extremely high or low pH. Some enzymes, such as those which act in the human stomach where the pH is very low, will have an appropriately low pH optimum. A buffer is a compound that will gain or lose H+ ions so that the pH changes very little.
Temperature: All chemical reactions speed up as the temperature is raised. As the temperature increases, more of the reacting molecules have enough kinetic energy to undergo the reaction. Since enzymes are catalysts for chemical reactions, enzyme reactions also tend to go faster with increasing temperature. However, if the temperature of an enzyme-catalyzed reaction is raised still further, an optimum is reached: above this point the kinetic energy of the enzyme and water molecules is so great that the structure of the enzyme molecules starts to be disrupted. The positive effect of speeding up the reaction is now more than offset by the negative effect of denaturing more and more enzyme molecules. Many proteins are denatured by temperatures around 40-50C, but some are still active at 70-80C, and a few even withstand being boiled.
In this exercise you will study the enzyme catalase, which accelerates the breakdown of hydrogen peroxide (a common end product of oxidative metabolism) into water and oxygen, according to the summary reaction:
2H 2 O 2 + Catalase —-> 2H 2 O + O 2 + Catalase
Catalase is found in animal and plant tissues, and is especially abundant in plant storage organs such as potato tubers, corms, and in the fleshy parts of fruits. You will isolate catalase from potato tubers and measure its rate of activity under different conditions.
The fact that one of the products of the above reaction (oxygen) forms a gas, it is convenient to observe the conversion from reactants to products. A small piece of filter paper can be soaked in a solution of catalase and immersed in a solution of hydrogen peroxide. It will immediately sink to the bottom of the vessel. As the catalase converts the hydrogen peroxide to water and oxygen, some of the oxygen gas accumulates on the disk, changing buoyancy of the disk and floating to the surface. The elapsed time from immersion to floatation is thus proportional to the rate of the reaction (the time required to produce sufficient product (O 2 ) to float the disk). This allows us to explore the effect of a number of variables on the rate of a reaction.
Part A : Extraction of Catalase
- Peel a fresh potato tuber and cut the tissue into small cubes. Weigh out 50 g of tissue.
- Place the tissue, 50 ml of cold distilled water, and a small amount of crushed ice in a pre-chilled blender.
- Homogenize for 30 seconds at high speed. From this point on, the enzyme preparation must be carried out in an ice bath.
- Filter the potato extract, then pour the filtrate into a 100-ml graduated cylinder. Add cold distilled water to bring up the final volume to 100 ml. Mix well. This extract will be arbitrarily labeled 100 units of enzyme per ml (100 units/ml). (NOTE – this will be enough catalase for all lab groups)
Work in groups of 4.
- Tape or permanent marker for labeling
- Five 50 ml beakers or large test tubes.
- Stock 3% H 2 O 2
- Catalase solution
- No2 Whatman filter paper
- Hole punchers
- Beaker with water
- Water baths at the following temperatures (4, 25, 37, 60 o C). These will be beakers on hot plates with temperature monitored by a thermometer.
PART B: Effect of Substrate Concentration
Before beginning, make a hypothesis and prediction about the scientific questions we are asking.
Q1. How does substrate concentration affect the speed at which catalase converts H 2 O 2 into water and oxygen?
Ha (Alternative hypothesis); H0 (Null hypothesis)
Write your scientific prediction (remember to use if—then—).
What are independent and dependent variables in this experiment?
- Place 20 ml stock 3% H 2 O 2 in one of the beakers or test tubes and label. Make sure your label identifies your group and concentration of H 2 O 2 .
- Dilute stock 3% H 2 O 2 to make 1.5%, 0.75%, 0.38% and 0.19%. You should figure out how to do this by serial dilution of your 3% H 2 O 2 stock. All beakers or test tubes should contain 10 ml except for the last beaker containing 0.19% H 2 O 2 .
- Make 25 small filter paper disks with a hole punch.
- With a forceps, grab a disk and immerse it in catalase solution for 3 seconds. BE PRECISE WITH THE TIME IT IS IMMERSED IN THE ENZYME. Immediately drop it into one of the solutions of H 2 O 2 and start your timer. If you look closely, you will see bubbles of O 2 forming on the disk. Stop your timer the moment the paper disk rises to the surface. Remove the paper disk and repeat the process four more times.
- Repeat step 4 for each of the concentration of H 2 O 2 .
- Record your data in a table.
DATA ANALYSIS
Graph your results by hand in your lab notebook. Plot the independent variables on X axis, dependent variable on Y axis. Use the averages of your replicates for the graph.
PART C: The effect of temperature on enzyme function and the reaction
Before beginning, make some hypothesis and predictions about the scientific questions we are asking.
Q1. How does temperature affect the speed at which catalase converts hydrogen peroxide into water and oxygen?
Ha (Alternative hypothesis)
H0 (Null hypothesis)
Write your scientific prediction here (remember to use if—then—)
- Prepare 5 test tubes with 10 ml each of 1.5% H 2 O 2 solution, and label them to identify your group and temperature. Keep one of your test tubes immersed in water at room temperature, the others in water baths at 4 o C (on ice), 37 o C, and 60 o C, respectively.
- Make 25 more filter paper disks.
- Measure the reaction rate as you did in part 1, by dipping each filter paper into catalase for 3 seconds, then recording the time it takes for a disk to rise to the top of each test tube after dropped to the bottom. As in part 1, repeat this procedure with 5 filter paper per test tube.
Post-lab Questions – Answer these questions in your lab notebook
- What were your conclusions regarding the effect of substrate concentration on catalase activity?
- What were your conclusions regarding the effect of temperature on catalase activity? Identify the optimum temperature on catalase activity, in other words, at what temperature was the amount of product produced by the enzyme-catalyzed reaction greatest ?
- If an enzyme were isolated from an organism, such as a clam, that lived in seawater that averages 14°C, what would you predict would be the optimal temperature for that enzyme, and why?
- Define enzyme denaturation in terms of protein structure.
LWTech General Biology (BIOL&160) Lab Protocols Copyright © by Lake Washington Institute of Technology is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book

IMAGES
VIDEO
COMMENTS
The two experiments that you conducted in Part A are summarized below. Tubes 1 through 4 are used to investigate the effect of temperature on enzyme activity. Tubes 4 and 5 are used to investigate the effect of denaturing an enzyme with heat and then using the enzyme at its normal temperature (body temperature).
The enzyme catalase quickly breaks down hydrogen peroxide into water and oxygen. In other words, catalase protects cells from the toxic effects of hydrogen peroxide. All aerobic cells produce catalase.
An experiment investigating the effect of pH on enzyme activity: How does pH affect the rate of catalytic reaction of catalase breaking down hydrogen peroxide?
Catalase is an enzyme, a large protein that speeds up the rate of a chemical reaction. The optimum pH level of catalase is between pH 7 and pH 11. At a pH level lower or higher than this range, the catalase stops working.
Enzyme Activity Using Toothpickase - simulate the activity of an enzyme by breaking toothpicks. Measure the effects of changes in temperature, pH, and enzyme concentration on reaction rates of an enzyme catalyzed reaction in a controlled experiment.
W e observed how three factors influence the activity of the enzyme catalase: enzyme concentration, pH, and temperature. Below are results from three lab sections. Averages are graphed in green. Catalase Reactions.
pH: To show students the impact of pH on enzyme efficiency, have them add a few drops of an acid and a base to the potato purees on a spot plate. Vinegar and bleach are great options. Repeat the experiment and have students determine at which pH catalase works best.
pH: pH is a logarithmic scale that measures the acidity or H+ concentration in a solution. As the pH is lowered, an enzyme will tend to gain H+ ions, and eventually enough side chains will be affected so that the enzyme’s shape is disrupted. Likewise, as the pH is raised, the enzyme will lose H+ ions and eventually lose its active shape.
In fact, there are thousands of different enzymes in your body that work around-the-clock to keep you healthy and active. In this science activity you will investigate one of these enzymes, called catalase, to find out how it helps to protect your body from cell damage.
Revise your understanding of enzymes, substrates, lock and key theory and the effect of temperature, substrate concentration and pH on reaction rate.