Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 26 September 2018

Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies
- Camille Lassale 1 , 2 ,
- G. David Batty 1 ,
- Amaria Baghdadli 3 , 4 ,
- Felice Jacka ORCID: orcid.org/0000-0002-9825-0328 5 ,
- Almudena Sánchez-Villegas 6 , 7 ,
- Mika Kivimäki ORCID: orcid.org/0000-0002-4699-5627 1 , 8 &
- Tasnime Akbaraly ORCID: orcid.org/0000-0002-2150-4190 1 , 3 , 9
Molecular Psychiatry volume 24 , pages 965–986 ( 2019 ) Cite this article
94k Accesses
457 Citations
1890 Altmetric
Metrics details
A Correction to this article was published on 04 March 2021
A Correction to this article was published on 21 November 2018
This article has been updated
With depression being the psychiatric disorder incurring the largest societal costs in developed countries, there is a need to gather evidence on the role of nutrition in depression, to help develop recommendations and guide future psychiatric health care. The aim of this systematic review was to synthesize the link between diet quality, measured using a range of predefined indices, and depressive outcomes. Medline, Embase and PsychInfo were searched up to 31 st May 2018 for studies that examined adherence to a healthy diet in relation to depressive symptoms or clinical depression. Where possible, estimates were pooled using random effect meta-analysis with stratification by observational study design and dietary score. A total of 20 longitudinal and 21 cross-sectional studies were included. These studies utilized an array of dietary measures, including: different measures of adherence to the Mediterranean diet, the Healthy Eating Index (HEI) and Alternative HEI (AHEI), the Dietary Approaches to Stop Hypertension, and the Dietary Inflammatory Index. The most compelling evidence was found for the Mediterranean diet and incident depression, with a combined relative risk estimate of highest vs. lowest adherence category from four longitudinal studies of 0.67 (95% CI 0.55–0.82). A lower Dietary Inflammatory Index was also associated with lower depression incidence in four longitudinal studies (relative risk 0.76; 95% CI: 0.63–0.92). There were fewer longitudinal studies using other indices, but they and cross-sectional evidence also suggest an inverse association between healthy diet and depression (e.g., relative risk 0.65; 95% CI 0.50–0.84 for HEI/AHEI). To conclude, adhering to a healthy diet, in particular a traditional Mediterranean diet, or avoiding a pro-inflammatory diet appears to confer some protection against depression in observational studies. This provides a reasonable evidence base to assess the role of dietary interventions to prevent depression. This systematic review was registered in the PROSPERO International Prospective Register of Systematic Reviews under the number CRD42017080579.
Similar content being viewed by others
Role of dietary factors in the prevention and treatment for depression: an umbrella review of meta-analyses of prospective studies

Adherence to the EAT-Lancet diet and incident depression and anxiety
Diet and depression: exploring the biological mechanisms of action
Introduction.
Depression, characterized by low mood, loss of interest or pleasure in life, and disturbed sleep or appetite, affects over 300 million people globally [ 1 ], which represents a global prevalence of 7% for women and 4% for men [ 2 ]. Depression is a leading cause of disease burden and a major contributor to global disability [ 3 ]. According to the World Health Organization, depressive and anxiety disorders cost the global economy $1 trillion in lost productivity each year [ 4 ].
Despite significant developments, conventional treatment is effective only in one in three cases of mood disorder [ 5 ]. Moreover, the condition is often recurrent, with relapse apparent in 50% of cases [ 6 ]. In this context, identifying modifiable risk factors to guide intervention strategies to prevent mood disorders and decrease their severity would appear to have value. A range of demographic, biological, genetic and behavioral determinants for depression have been proposed [ 7 , 8 , 9 , 10 ]. Of the latter, habitual diet has been increasingly examined as a potential independent predictor of disease risk (e.g., [ 11 ]) and the dietary intake of specific nutrients such as n-3 polyunsaturated fatty acids, B vitamins, zinc, and magnesium have been implicated in brain function [ 9 , 12 , 13 , 14 , 15 ]. The neurological pathways potentially affecting depression risk that can be modulated by nutritional intake are related to inflammation, oxidative stress, neuroplasticity, mitochondrial function, and the gut microbiome [ 9 ].
The major challenge in understanding the role of diet in the etiology of chronic disease, including depression, is the characterization of this complex exposure. One approach is the identification of dietary patterns in the population under study by statistical modeling such as factor analysis (empirically-derived dietary patterns). These dietary patterns closely match the dietary habits of the studied population but do not necessarily reflect an optimal diet and are hardly replicable to other populations. In contrast to these a posteriori methods, a priori methods generate dietary indices based on existing knowledge of what constitutes a “healthy” diet (hypothesis-oriented dietary patterns). Based on a limited set of specific food groups, rather than specific nutrients, dietary indices reflecting adherence to an ideal diet can be very useful for clinicians to communicate with patients. Recent reviews have shown that healthy dietary patterns are associated with a decreased risk of depression or depressive disorders [ 16 , 17 , 18 , 19 , 20 ], but these are not universal findings [ 16 ]. Additionally, comparability of the studies is hampered by the variability in methodology and combination of estimates obtained using both hypothesis-oriented and data-driven dietary patterns. There is only one review of literature using a priori defined scores based on adherence to a traditional Mediterranean diet in relation to depression [ 21 ]. However, no formal comparison of the Mediterranean diet score exists with other widely used diet quality scores as, to the best of our knowledge, there is no exhaustive review of all a priori diet quality indices.
Accordingly, we provide a systematic review of studies assessing whether adherence to various dietary guidelines or traditional dietary patterns is associated with depressive symptoms and depression. For each dietary index we conducted a meta-analysis of results from observational studies. With the relationship likely to be bidirectional—mood can induce changes in eating behavior—only longitudinal studies can clarify the direction of the association. We therefore stratified our results according to study design.
This systematic review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement [ 22 ] and was registered in the PROSPERO International Prospective Register of Systematic Reviews (# CRD42017080579 at www.crd.york.ac.uk/PROSPERO ).
Search strategy
We used a four-pronged approach to identifying relevant publications. First, we searched Medline, Embase and PsychINFO via Ovid ( http://www.ovid.com ) for articles published since their inception (1946) to 31 st May 2018. The following keywords and index terms were used (“depression” or “depress* symptom*”) AND (“diet*”) AND (“index*” or “score*” or “pattern*” or “quality”). The search was limited to articles published in the English language and to full-text articles (conference abstracts were not considered). Second, we scrutinized the reference sections of the retrieved articles and systematic reviews. Third, we contacted experts in the field. Fourth, we searched our own files. The search was conducted in parallel by two authors (TA and CL) with any disagreements resolved by a third (AB).
Study selection
To be included in this systematic review, articles had to meet the following criteria: (1) Exposure: comprehensive dietary assessment (food frequency questionnaire, 24-h diet recall, food record, diet history) and use of an a priori dietary score or index; (2) Outcome: clinical depression assessed by the study staff, medical records or self-reported clinician-diagnosed (e.g., “Have you ever been diagnosed with depression by a medical doctor?”), depressive symptoms assessed by validated scales/questionnaires (such as the Center for Epidemiological Studies Depression scale, CES-D), and defined according to validated cutoffs of these scales (i.e., cutoff of 16 for the 20-item CES-D scale) and the use of anti-depressive drugs considered only when it was combined to clinical depression or depressive symptoms assessment; (3) Design: observational study (cross-sectional, cohort, case-control); (4) Population: general free-living populations without any age limit that includes outpatients (individuals not hospitalized for physical or mental health reasons) and non-institutionalized individuals. If the study had any of the following characteristics, it was excluded: (1) Exposure: no measure of whole diet (e.g., dietary screeners or individual questions) or use of a posteriori dietary patterns; (2) Outcome: bipolar disorders, overall mood states, psychosocial stressors or perceived stress; (3) Design: intervention studies; (4) Population: pregnant or lactating women, inpatient/hospitalized populations.
Data extraction
After study selection, the following information was extracted from each retrieved article: first author’s surname, journal, year published, geographical location, study design, follow-up time (if applicable), sex and mean age, sample size, number of cases, dietary assessment tool, dietary score used (range and mean score), assessment of depression, depressive symptoms scale and threshold used (if symptoms), modeling strategy, confounders used, main findings including odds ratios or hazard/risk ratios and their standard errors/confidence intervals. When a study provided several estimates, we chose to use those from the most complex model (that is, the one including the largest number of confounders).
Quality and risk of bias assessment
We adapted the Newcastle-Ottawa checklist [ 23 ] to assess whether cohorts were representative of the wider population (as opposed to a selected occupational group, for instance), if diet was ascertained by means of a validated dietary assessment tool (e.g., FFQ), if the dietary score used was validated, whether follow-up was sufficient to preclude reverse causation (≥5 years), and if appropriate statistical adjustment was made (age, sex, smoking, physical activity, body mass index, total energy intake). If at least four of the five of the above criteria was met, the study was considered of high quality and to be at low risk of bias.
Statistical analysis
For each dietary score (exposure variable), we conducted separate meta-analyses dependent on study design (cross-sectional vs. longitudinal). We combined the results of the studies that presented analyses with the dietary score as a categorical variable, computing forest plots and combined odds, hazard or risk ratios for depression for the healthiest compared to the least healthy category. When the dietary score was analyzed as a continuous variable (four studies [ 24 , 25 , 26 , 27 ]), the estimates were not included in the calculation of the combined results. Estimates (beta and standard error) from studies that used depressive symptoms (outcome) as a continuous variable were converted into log odds ratios by multiplying by a factor 1.81 and then exponentiated [ 28 ]. We used random-effect meta-analysis models to account for potential heterogeneity and assessed heterogeneity by the I 2 statistic [ 29 ]. Potential for publication bias was examined using contour-enhanced funnel plots where asymmetry and absence of studies in areas of non-significance suggest the presence of reporting bias [ 30 ]. We also calculated the Egger and Begg test for small study effect. Stata version 14 (StataCorp, TX, USA) was used for the statistical analyses.
Code availability
The Stata commands metan, confunnel and metabias were used. All codes used to generate the meta-analysis results can be obtained from the authors upon request.
Sensitivity analyses
As results are stratified by dietary index and by study design, further stratification can lead to only one association estimate in some strata. Therefore, we only present the sensitivity analyses results in the subgroups that contain the majority of studies. To test the impact of outcome definition (clinical depression vs. depressive symptoms), of age of the participants, of geographical region (high vs. low-middle income countries) and of study quality, we performed sensitivity analyses by variously excluding: the studies using clinical depression as they were a minority; studies on adolescents; studies performed in low-middle income countries; and studies of low quality. Finally, a study using the Mediterranean diet was identified that used psychological distress as a marker of depression [ 31 ], so we also performed a sensitivity analysis by excluding this study for a more strict assessment of depression.
In Supplemental Fig. 1 we depict the process of study selection. The search yielded 3272 records (after exclusion of duplicates), of which 3058 were excluded after title and abstract screening. Out of the 214 full-text articles assessed for eligibility, we retained 51 that we scrutinized for methodological quality. The articles excluded are listed by reason of exclusion in Supplemental material . A total of ten articles were dropped after methodological quality check, comprising the presentation of only unadjusted comparisons between groups and no further statistical modeling in six studies [ 32 , 33 , 34 , 35 , 36 , 37 ], or no measure of whole diet in a further four [ 38 , 39 , 40 , 41 ]. This yielded a total 41 articles for this systematic review: 20 longitudinal and 21 cross-sectional studies.
The majority of studies were on generally healthy participants. Two studies involved participants at high risk of knee osteoarthritis [ 42 , 43 ] and one included participants with a history of myocardial infarction [ 44 ]. Ten analyses used a Mediterranean diet score [ 25 , 26 , 27 , 31 , 43 , 45 , 46 , 47 , 48 , 49 ], seven the Healthy Eating Index (HEI) or the Alternative Healthy Eating Index (AHEI) [ 48 , 50 , 51 , 52 , 53 , 54 , 55 ], four a Dietary Approaches to Stop Hypertension (DASH) diet score [ 56 , 57 , 58 , 59 ], nine the Dietary Inflammatory Index (DII) [ 42 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 ], and 15 a variety of scores such as adherence to national dietary guidelines or general “diet quality” [ 44 , 46 , 51 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 ]. The components included in each of the main diet scores (Mediterranean, HEI, AHEI, DASH) are summarized in Supplemental Table 1 . One study simultaneously captured three scores, a Mediterranean diet score, the HEI, and a pro-vegetarian dietary pattern [ 48 ], another compared the Mediterranean diet score with the Australian Recommended Food Score [ 46 ], and a last one compared the AHEI with three other scores [ 51 ].
We graded 32 analyses as being of high quality (scoring four or five) [ 25 , 26 , 27 , 31 , 42 , 45 , 46 , 48 , 49 , 51 , 55 , 56 , 57 , 58 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 69 , 71 , 72 , 73 , 74 , 75 , 77 , 79 ], whereas 12 studies had a low quality score of three or less [ 43 , 44 , 47 , 48 , 50 , 52 , 53 , 54 , 59 , 70 , 76 , 78 ], of which the majority (nine) were cross-sectional studies.
Mediterranean diet
Adherence to a traditional Mediterranean diet was measured by four different indices: the original Mediterranean Diet Score (MDS) [ 25 , 31 , 46 , 48 ] developed by Trichopoulou and colleagues [ 80 ], the relative Mediterranean diet score (rMED) [ 45 ], the alternative Mediterranean diet score (aMED) [ 26 , 27 , 43 ] or the Mediterranean Style Dietary Pattern Score (MSDPS) [ 49 ]. The MDS and rMED include nine items: five beneficial (fruit, vegetable, legumes, cereals, fish), two considered detrimental (meat, dairy), one component on fat (mono-unsaturated fatty acids/saturated fatty acids [MDS] or olive oil intake [rMED]) and one component on moderate alcohol intake. The MDS ranges from zero to nine points: one point is allocated if the intake is above the median, zero if below (or inversely for detrimental items). The rMED is based on tertiles as cutoffs, therefore ranges 0–18. The aMED scores from zero (lower adherence) to five (better adherence) on 11 components (same as MDS, adding poultry [detrimental] and potatoes [beneficial]), so the total score ranges 0–55. The MSDPS comprises 13 components (same as aMED, adding sweets and eggs), each scored continuously from 0 to 10 and the total score is standardized, ranging 0–100.
The study characteristics are given in Table 1 . There were two reports from the same study (the Spanish Seguimiento Universidad de Navarra, SUN [ 47 , 48 ]), so we included the one with the longest follow-up (8.5 years) [ 48 ]. In total, we considered data from six cohort studies comprising samples drawn from France [ 45 ], Australia [ 31 , 46 ], Spain [ 48 ], the UK [ 25 ] and the US [ 27 ] (average 9.1 years of follow-up). There were three cross-sectional studies (the US [ 43 ], Greece [ 26 ] and Iran [ 49 ]).
The combined estimate from four longitudinal studies [ 31 , 45 , 46 , 48 ] shows that people in the highest category of adherence to a Mediterranean diet have lower odds/risk of incident depressive outcomes, with an overall estimate of 0.67; 95% confidence interval (CI): 0.55, 0.82 compared to people with lowest adherence (Fig. 1 ). The estimate from two studies [ 25 , 27 ] were produced using linear models or generalized estimating equation and therefore not comparable to the other studies: one showed a significant inverse association over time [ 27 ], and the other study, on adolescents, found no significant association [ 25 ]. The three cross-sectional studies yielded inconsistent results.
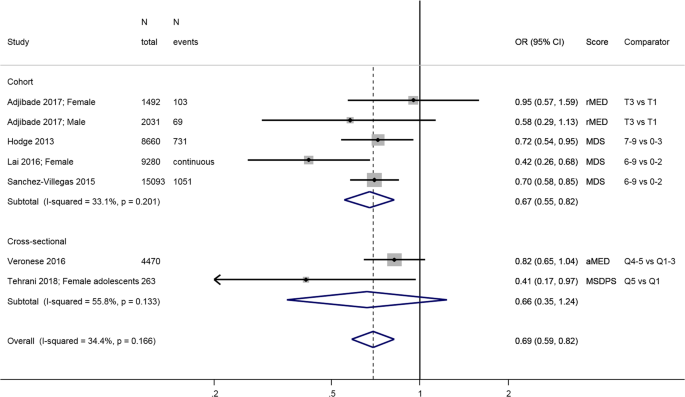
Meta-analysis of studies investigating the association between a traditional Mediterranean diet and depressive outcomes. Estimates are ORs, RRs or HRs of depression for people with highest adherence compared to lowest adherence (categories or quantiles specified). MDS Mediterranean diet score, rMED relative MDS, aMED alternative MDS, T tertile, Q quintile
Healthy Eating Index (HEI)
There were three longitudinal cohort studies (United Kingdom [ 55 ], Spain [ 48 ], and France [ 51 ]) with an average 6.5 years follow-up) and four cross-sectional studies (US [ 50 , 52 ] and Iran [ 53 , 54 ]) that used either the HEI-2005 [ 50 , 52 ], the original AHEI [ 55 ] or the AHEI-2010 [ 48 , 51 , 53 , 54 ] (Table 1 ) . The HEI-2005 is based on the Dietary Guidelines for Americans 2005, ranges 0–100 and has 12 components, each scoring five or ten points: total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, total grains, whole grains, dairy, meat and beans, oils, saturated fat, sodium, empty calories. The AHEI includes nine components, each with a score of up to ten points except multivitamin use (vegetables, fruit, nuts and soy protein, ratio of white to red meat, cereal fiber, trans fat, polyunsaturated-to-saturated fat ratio, duration of multivitamin use, and alcohol), for a total score ranging 2.5 to 87.5. Finally, the AHEI-2010 comprises 11 items (vegetables, fruit, nuts and legumes, red/processed meat, whole grains, trans fat, long-chain (n-3) fatty acids, polyunsaturated fat, alcohol, sugar-sweetened beverages and fruit juice, and sodium) and ranges 0–110.
The three longitudinal studies [ 48 , 51 , 55 ] show a lower risk of incident depression in the high diet score category compared to low (0.76; 95% CI: 0.57, 1.02), but this association is only borderline significant at the conventional level (Fig. 2 ). There was large heterogeneity in the estimates of these three studies ( I 2 = 80.7%, p = 0.001). Overall, the cross-sectional studies show an inverse association between HEI-2005 or AHEI-2010 and prevalence of depression: OR = 0.53; 95% CI: 0.38, 0.75, with no apparent heterogeneity ( I 2 = 32.1%, p = 0.22) (Fig. 2 ).
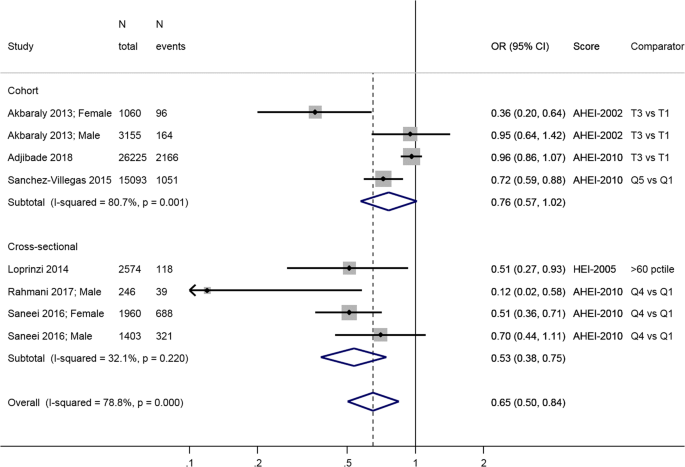
Meta-analysis of studies investigating the association between HEI/AHEI and depressive outcomes. Estimates are ORs, RRs, or HRs of depression for people with highest adherence compared to lowest adherence (categories or quantiles specified). HEI healthy eating index, AHEI Alternatative Heatlhy Eating Index, T tertile, Q5 quintile, Q4 quartile, 60pctile 60 th percentile
Dietary Approaches to Stop Hypertension (DASH)
Four studies [ 56 , 57 , 58 , 59 ] used the DASH diet score developed by Fung and colleagues [ 81 ] or a modified version (Table 1 ). It comprises eight components relative to food group intakes (negative: sweet beverages, meat, sodium; positive: fruit, vegetables, legumes and nuts, wholegrain, low-fat dairy), scores of one to five correspond to sex-specific quintiles, and the total sum score ranges 8–40.
Investigators in the only longitudinal study, the Spanish SUN cohort [ 58 ], compared the Fung DASH diet score to three other DASH scores [ 82 , 83 , 84 ] which use different scoring system or include nutrient intakes, and found a significant negative association with depression incidence only when using the Fung score; the other DASH scores were not associated with clinical depression (Fig. 3 ). Results from cross-sectional studies reveal no association with the exception of an Iranian study of adolescent girls [ 56 ] that found an inverse association between DASH and depressive symptoms. Overall, the link between adherence to a DASH diet and depression has been little studied and results are inconclusive, particularly in adults.
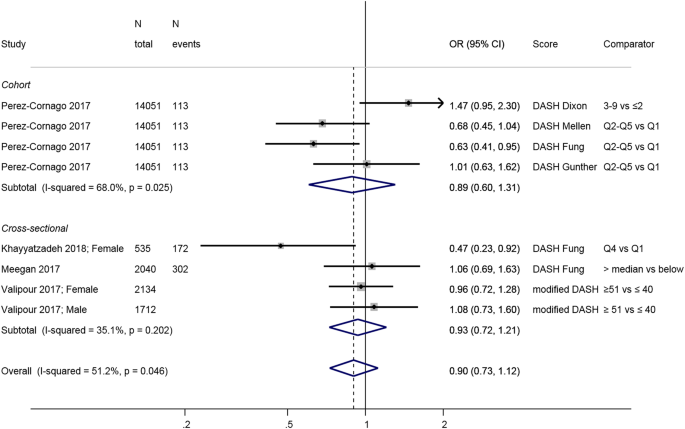
Meta-analysis of studies investigating the association between a DASH diet and depressive outcomes. Estimates are ORs, RRs, or HRs of depression for people with highest adherence compared to lowest adherence (categories or quantiles specified). DASH dietary approaches to stop hypertension, T tertile, Q5 quintile, Q4 quartile
Dietary Inflammatory Index (DII)
The DII is a literature-derived, population-based index that aims to quantify the overall effect of diet on inflammatory potential based on the individual inflammatory effects of up to 45 food parameters [ 85 ]. We found five cohort studies from the UK [ 61 ], the US [ 42 ], France [ 60 ], Australia [ 65 ] and Spain [ 67 ] and four cross-sectional studies from the US [ 62 , 66 ], Ireland [ 63 ] and Iran [ 64 ] (Table 1 ).
Comparing the least inflammatory to the most inflammatory diet, there was a combined inverse association in both longitudinal (overall HR = 0.76; 95% CI: 0.63, 0.92) and cross-sectional (overall OR = 0.64; 95% CI: 0.45, 0.91) (Fig. 4 ) analyses. There was significant heterogeneity in the results from both longitudinal ( I 2 = 55.3%, p = 0.04) and cross-sectional studies ( I 2 = 69.0%, p = 0.006), in particular due to differences between estimates in men and women, with three studies showing a negative association in women but no relationship in men [ 61 , 63 , 66 ]; another study found the reverse [ 60 ].
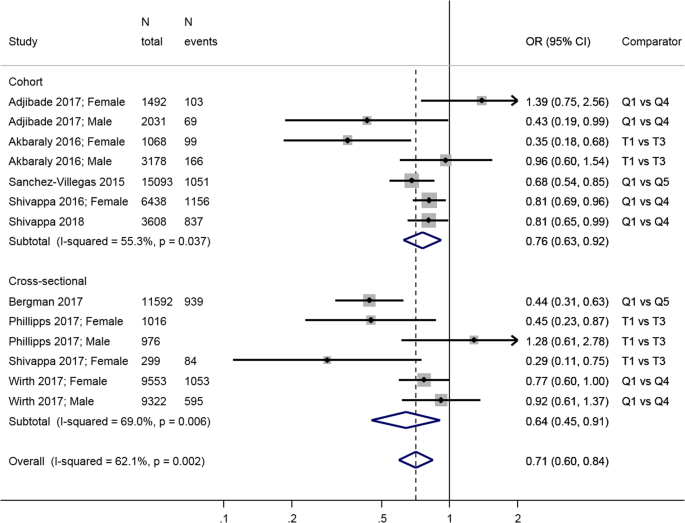
Meta-analysis of studies investigating the association between the Dietary Inflammatory Index DII and depressive outcomes. Estimates are ORs, RRs, or HRs of depression for people with lowest adherence compared to highest adherence (categories or quantiles specified). T tertile, Q5 quintile, Q4 quartile
Other dietary indices
A variety of other scores were used to describe adherence to national dietary guidelines [ 44 , 46 , 51 , 69 , 71 , 73 , 75 , 77 , 79 ], to the American Heart Association recommendations [ 70 ], and pro-vegetarian [ 67 ] or general “diet quality” scores [ 2 , 72 , 74 , 76 , 78 ] (Table 1 ). Owing to an absence of comparability, we show all estimates on a summary plot (Fig. 5 ) but do not provide an overall estimate. We observed a trend towards an inverse association between higher diet quality and depression.
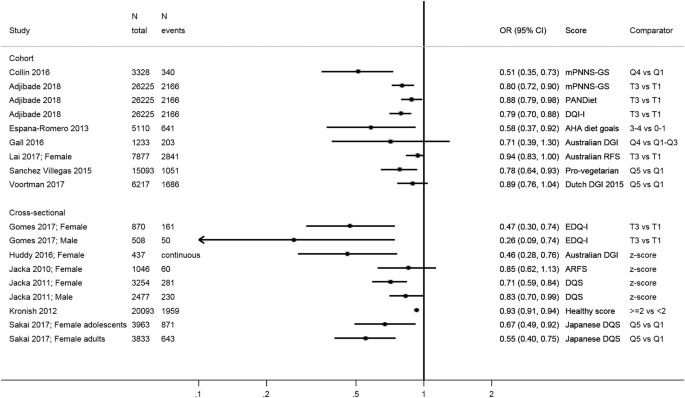
Summary of studies investigating the association between various other diet quality scores and depressive outcomes. mPNNS-GS modified score of adherence to the French dietary guidelines (PNNS), AHA American Heart Association, (A)RFS (Australian) Recommended Food Score, DGI Dietary Guidelines Index, DQI-I Diet Quality Index International, DQS Diet quality score, EDQ-I Elderly Dietary Quality Index, PANDiet Diet Quality Index Based on the Probability of Adequate Nutrient Intake, T tertile, Q5 quintile, Q4 quartile
Risk of bias
We present the contour-enhanced funnel plots for the four main dietary scores on Supplemental Fig. 2 . There was little evidence of publication bias as evidenced by visual inspection of the plots: estimates from the included studies are distributed equally around the overall estimate for each index used, and studies with both significant and non-significant estimates were included. Egger’s test for small study effects was significant only for the studies using the HEI or AHEI ( p = 0.01), but the Begg test was non-significant ( p = 0.39). All tests for small study effects were non-significant for Mediterranean, DASH and DII.
Regarding outcome definition, when analyzing only studies on depressive symptoms outcomes (that is, excluding studies using clinical depression as outcome), the results remained similar for the MDS and DII (Supplemental Figs. 3 and 5 respectively), but the overall estimate for the HEI /AHEI based on three prospective studies was substantially attenuated (Supplemental Fig. 4 ): 0.74; 95% CI: 0.47, 1.18. Results on clinical depression all come from the Spanish SUN cohort, which showed strong prospective associations with the MDS, HEI, and DII scores [ 48 , 67 ] but not consistent with different DASH scores [ 58 ]. In addition, Supplemental Figure 6 shows that for all other dietary scores, significant negative associations were reported with depressive symptoms, whereas the three studies that used clinical depression report non-significant associations [ 71 , 75 , 79 ]. Regarding study quality, all cross-sectional studies using HEI/AHEI were judged of low quality; therefore, when limiting the evidence to high quality studies, this dietary score only shows a weak overall estimate from three cohort studies. When assessing the differences by geographical region, we found that studies in middle-income country were all conducted in Iran. Two of them, carried out in adults, assessed the cross-sectional association between the HEI and depressive symptoms and reported similar estimates to those observed in an Irish cross-sectional study using the HEI score too. In addition, three Iranian studies were carried out on adolescents and found significant inverse associations between Mediterranean diet, DASH and DII scores and depressive symptoms. The global estimates between each of these dietary scores and depressive symptoms were not altered after excluding studies conducted in adolescents as illustrated in Supplemental Figure 7 , 8 and 9 , showing results for the Mediterranean diet, DASH, and DII scores respectively. Finally, having excluded the study using psychological distress [ 31 ], the overall results of the association between a Mediterranean diet and depressive outcomes remained essentially unchanged (Supplemental Figure 10 ).
Main findings
By focusing on dietary indices, this systematic review is the first to provide an exhaustive overview of the evidence linking a wide range of comparable a priori diet quality indices and depressive outcomes. From analyses of longitudinal studies, there is a robust association between both higher adherence to a Mediterranean diet and lower adherence to a pro-inflammatory diet and a lower risk of depression. While there are fewer studies, the same trend seems apparent for indices such as the Healthy Eating Index and several other country-specific dietary guidelines scores.
Comparison with the literature
According to recent reviews, the available evidence suggests an inverse association between healthy or prudent dietary patterns and depression, despite some inconsistent results and heterogeneity in the methods used to define healthy dietary patterns [ 16 , 17 , 18 , 19 , 20 ]. The beneficial effect of the Mediterranean diet has been reviewed in 2013 [ 21 ] but these studies were, with one exception, cross-sectional. The addition of recent longitudinal studies reinforces the previous review in concluding that highest compared to lowest adherence to a Mediterranean diet is associated with lower risk of incident depressive outcome. A unique feature of the present review is the inclusion and comparison of a wide range of a priori dietary scores. We found that the measures of adherence to dietary guidelines that have been commonly studied in relation to other chronic diseases and mortality, HEI and AHEI [ 86 , 87 , 88 ], also show encouraging results in relation to depression; however, more longitudinal studies are needed to confirm the direction of the associations.
Biological mechanisms
As summarized in Supplemental Table 1 , the dietary scores share common elements: higher fruits, vegetables, and nut intake, lower intakes of pro-inflammatory food items such as processed meats and trans fats, and alcohol in moderation. To date, a number of factors have been proposed to cause diet-induced damage to the brain, including oxidative stress, insulin resistance, inflammation, and changes in vascularization, as all these factors can be modified by dietary intake and have been associated with occurrence of depression [ 89 ]. Moreover, recent human studies [ 90 ] support extensive pre-clinical research [ 91 ], suggesting an impact of diet on the hippocampus. Fruit, vegetables, nuts, and wine in moderation have been associated with better metabolic health outcomes [ 92 , 93 ], which share a common etiology with depression [ 94 ]. Those foods have antioxidant and anti-inflammatory properties. Protection against oxidation can reduce neuronal damage due to oxidative stress [ 17 ]. Systemic inflammation can affect the brain by active transport of cytokines through the brain endothelium or activation of vagal fibers, and also plays a role in the regulation of emotions through mechanisms involving neurotransmitters including serotonin, dopamine, noradrenaline, and glutamate [ 60 ]. Our results show a consistent association between an inflammatory diet (measured by the DII) and incident depressive outcomes, which supports the hypothesis that avoiding pro-inflammatory foods in favor to anti-inflammatory diet might contribute to prevent incidence of depression and depressive symptoms. Finally, an extensive body of evidence now points to the microbiome-gut-brain axis as playing a key role in neuropsychiatry, and to the primacy of diet as a factor modulating this axis [ 95 ].
Limitations
With depression being the psychiatric disorder incurring the largest societal costs in Europe, our study is part of an effort to gather evidence on the role of nutrition in depression, to help develop recommendations and guide future psychiatric health care. Recent reviews [ 16 , 17 , 18 , 19 ] paved the way and our results come to complement the previous studies by focusing on dietary indices that can have a direct application in clinical settings.
However, there are various methodological considerations that need to be taken into account when interpreting the results of this meta-analysis. First, despite defining strictly our outcome of interest to unipolar depression or depressive symptoms, there was heterogeneity across studies: most used questionnaires, in particular the CES-D, although differing versions, and some questionnaires were only used in a single study (MFQ [ 25 ], BDI [ 56 ]). Only a minority of studies examined clinical depression [ 48 , 58 , 67 , 71 , 75 , 79 ], assessed by clinical interview or self-reported physician diagnosis, complemented by the use of anti-depressants. Therefore, the accuracy of the prevalence or incidence may differ from one study to another. Moreover, depressive symptoms may be transient and are potentially reversible, whereas diagnosed depression is of greater severity, although they can also be reversible and transient. Our results show that most studies focused on depressive symptoms, but there is a lack of evidence for clinical depression. The only studies included in the present review that used formal diagnosis of depression as an outcome are from the SUN cohort, which showed strong and robust associations with four different dietary indices [ 48 , 67 ] except for DASH scores [ 58 ], and three other studies that found no significant association with the Australian [ 71 , 75 ] or with the Dutch [ 79 ] dietary guidelines scores. The meta-analysis conducted by Molendijk et al. found that there was no overall association between adherence to healthy dietary patterns and incidence of depression using a formal diagnosis as outcome [ 16 ], but this review included only three studies, one of which used internalizing disorder (not specifically depression) in children as outcome, so the comparability of these three estimates is problematic.
Second, even under the same diet index name, the operationalization differed between studies depending on the dietary data available and how they were collected. It is common for large observational studies to collect self-reported dietary data with imperfect instruments such as food frequency questionnaires. These are associated with substantial measurement error, which can reduce the ability to detect associations [ 96 ]. Moreover, the majority of studies assessed diet at a single time point and did not take into account possible changes in diet quality over time that may be concomitant to the development of depressive symptoms. Furthermore, most studies compare extreme categories, e.g., top vs. bottom, but some used tertiles, others quartiles, or quintiles, making the comparisons less straightforward. In contrast to other meta-analyses [ 16 , 17 ], we presented global estimates of studies assessing the same dietary score, analyzed as categorical variable, and did not include studies assessing dietary scores as continuous variables, in order to provide more homogeneous results and an accurate quantification of the diet-depression relationship. Additionally, by presenting separate estimates according to the cross-sectional and longitudinal design of studies with long-term follow-up that should preclude reverse causality, and finding comparable results in both designs for most indices, our study provides support for an effect of overall diet on depression outcomes.
Third, we only included studies that used statistical adjustment (as opposed to simple mean comparisons between groups for instance). However, given the heterogeneity in the assessment of covariates, the comparability between studies is limited. All studies took age, sex (when relevant), energy intake and sociodemographic factors into account and the majority also included lifestyle factors and cardiometabolic markers; however, many of these used heterogeneous measures: some only adjusted for BMI, whereas others had a full cardiometabolic profile (blood pressure, cholesterol, diabetes, etc.). Also, a few studies did not include smoking nor physical activity [ 27 , 52 , 71 , 76 ], which are common correlates to diet quality and therefore the “effect” of diet quality observed in these studies may be a proxy of a healthy overall lifestyle. However, most studies do adjust for other health behaviors and the weight of the data suggests that the relationship between diet quality and depression is independent of other health behaviors, as well as income, education, and body weight. The majority of longitudinal analyses investigated incident depression, i.e., excluded prevalent depression at baseline, except three that only adjusted for baseline symptoms/use of antidepressants [ 25 , 46 , 71 ]. Some cross-sectional studies included anti-depressant use or history of depression as covariates [ 44 , 54 , 57 , 59 , 63 , 78 ], but no clear trend was observed in terms of effect size or significance of the estimates produced when comparing these with the studies that did not.
Fourth, the vast majority of the studies included in this systematic review were conducted in high income countries (Australia, France, Greece, Ireland, Japan, Netherlands, Norway, Spain, UK, US), with only seven studied in low-and-middle income countries (Brazil [ 72 ] and Iran [ 49 , 53 , 54 , 56 , 59 , 64 ]. Hence, the generalizability of the findings to low-and-middle income countries is limited. Moreover, we did not want to include an age limit and our systematic review includes three studies on adolescents [ 25 , 49 , 64 ]. Psychological disorders may express differently during adolescence as opposed to later in life, but the exclusion of those studies did not change the overall results and conclusions. Therefore, considering all the above limitations, extra care should be applied when using and interpreting the meta-analysis estimates.
Conclusions
Our review shows that there is observational evidence to suggest that both adhering to a healthy diet, in particular a traditional Mediterranean diet, and avoiding a pro-inflammatory diet is associated with reduced risk of depressive symptoms or clinical depression. That the majority of recovered studies were cross-sectional in design, with the problem of reverse causality being acute in the context of diet and depression, there is a clear need for more prospective studies. Moreover, while recent intervention studies provide preliminary evidence [ 97 , 98 ], further well-powered clinical trials are required to assess the role of dietary patterns in the prevention of onset, severity, and recurrence of depressive episodes.
Change history
21 november 2018.
This article was originally published under standard licence, but has now been made available under a CC BY 4.0 license. The PDF and HTML versions of the paper have been modified accordingly.
04 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41380-021-01056-7
World Health Organization. Depression fact sheet 2018 [Available from: http://www.who.int/mediacentre/factsheets/fs369/en/ .
Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int J Epidemiol. 2014;43:476–93.
Article PubMed PubMed Central Google Scholar
Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59.
Article Google Scholar
World Health Organization. Mental health in the workplace 2017 [Available from: http://www.who.int/mental_health/world-mental-health-day/2017/en/ .
van Zoonen K, Buntrock C, Ebert DD, Smit F, Reynolds CF 3rd, Beekman AT, et al. Preventing the onset of major depressive disorder: a meta-analytic review of psychological interventions. Int J Epidemiol. 2014;43:318–29.
Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27:959–85.
Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–75.
Article CAS PubMed Google Scholar
Yang L, Zhao Y, Wang Y, Liu L, Zhang X, Li B, et al. The effects of psychological stress on depression. Curr Neuropharmacol. 2015;13:494–504.
Article CAS PubMed PubMed Central Google Scholar
Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. 2017 Nov;76:427–436.
Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018 Jul 1;175:631–648.
Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. Br J Psychiatry. 2009;195:408–13.
Gomez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–78.
Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10:377–85.
CAS PubMed Google Scholar
Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2: macronutrients. J Nutr Health Aging. 2006;10:386–99.
Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012;17:1272–82.
Molendijk M, Molero P, Ortuno Sanchez-Pedreno F, Van der Does W, Martinez-Gonzalez MA. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2017;226:346–54.
Article PubMed Google Scholar
Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99:181–97.
Khalid S, Williams CM, Reynolds SA. Is there an association between diet and depression in children and adolescents? A systematic review. Br J Nutr. 2016;116:2097–108.
Li Y, Lv MR, Wei YJ, Sun L, Zhang JX, Zhang HG, et al. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017;253:373–82.
Rahe C, Unrath M, Berger K. Dietary patterns and the risk of depression in adults: a systematic review of observational studies. Eur J Nutr. 2014;53:997–1013.
Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74:580–91.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Beydoun MA, Wang Y. Pathways linking socioeconomic status to obesity through depression and lifestyle factors among young US adults. J Affect Disord. 2010;123:52–63.
Winpenny EM, van Harmelen AL, White M, van Sluijs EM, Goodyer IM. Diet quality and depressive symptoms in adolescence: no cross-sectional or prospective associations following adjustment for covariates. Public Health Nutr. 2018 Sep;21:2376–2384.
Mamplekou E, Bountziouka V, Psaltopoulou T, Zeimbekis A, Tsakoundakis N, Papaerakleous N, et al. Urban environment, physical inactivity and unhealthy dietary habits correlate to depression among elderly living in eastern Mediterranean islands: the MEDIS (MEDiterranean ISlands Elderly) study. J Nutr Health Aging. 2010;14:449–55.
Skarupski KA, Tangney CC, Li H, Evans DA, Morris MC. Mediterranean diet and depressive symptoms among older adults over time. J Nutr Health Aging. 2013;17:441–5.
Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–31.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
Hodge A, Almeida OP, English DR, Giles GG, Flicker L. Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. Int Psychogeriatr. 2013;25:456–66.
Exebio JC, Zarini GG, Exebio C, Huffman FG. Healthy Eating Index scores associated with symptoms of depression in Cuban-Americans with and without type 2 diabetes: a cross sectional study. Nutr J. 2011;10:135.
Forsyth AK, Williams PG, Deane FP. Nutrition status of primary care patients with depression and anxiety. Aust J Prim Health. 2012;18:172–6.
Hernandez-Galiot A, Goni I. Adherence to the Mediterranean diet pattern, cognitive status and depressive symptoms in an elderly non-institutionalized population. Nutr Hosp. 2017;34:338–44.
Kuczmarski MF, Cremer Sees A, Hotchkiss L, Cotugna N, Evans MK, Zonderman AB. Higher Healthy Eating Index-2005 scores associated with reduced symptoms of depression in an urban population: findings from the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) study. J Am Diet Assoc. 2010;110:383–9.
Quehl R, Haines J, Lewis SP, Buchholz AC. Food and mood: diet quality is inversely associated with depressive symptoms in female university students. Can J Diet Pract Res.2017;78:124–8.
Tangney CC, Young JA, Murtaugh MA, Cobleigh MA, Oleske DM. Self-reported dietary habits, overall dietary quality and symptomatology of breast cancer survivors: a cross-sectional examination. Breast Cancer Res Treat. 2002;71:113–23.
Bertoli S, Spadafranca A, Bes-Rastrollo M, Martinez-Gonzalez MA, Ponissi V, Beggio V, et al. Adherence to the Mediterranean diet is inversely related to binge eating disorder in patients seeking a weight loss program. Clin Nutr. 2015;34:107–14.
Jacka FN, Kremer PJ, Leslie ER, Berk M, Patton GC, Toumbourou JW, et al. Associations between diet quality and depressed mood in adolescents: results from the Australian Healthy Neighbourhoods Study. Aust NZ J Psychiatry. 2010;44:435–42.
Jacka FN, Rothon C, Taylor S, Berk M, Stansfeld SA. Diet quality and mental health problems in adolescents from East London: a prospective study. Social Psychiatry Psychiatr Epidemiol. 2013;48:1297–306.
Pagliai G, Sofi F, Vannetti F, Caiani S, Pasquini G, Molino Lova R, et al. Mediterranean diet, food consumption and risk of late-life depression: the Mugello Study. J Nutr Health Aging. 2018;22:569–574.
Article CAS Google Scholar
Shivappa N, Hebert JR, Veronese N, Caruso MG, Notarnicola M, Maggi S, et al. The relationship between the dietary inflammatory index (DII) and incident depressive symptoms: a longitudinal cohort study. J Affect Disord. 2018;235:39–44.
Veronese N, Stubbs B, Noale M, Solmi M, Luchini C, Maggi S. Adherence to the Mediterranean diet is associated with better quality of life: data from the osteoarthritis initiative. Am J Clin Nutr. 2016;104:1403–9.
Rius-Ottenheim N, Kromhout D, Sijtsma FPC, Geleijnse JM, Giltay EJ. Dietary patterns and mental health after myocardial infarction. PLoS ONE [Electron Resour]. 2017;12:e0186368.
Adjibade M, Assmann KE, Andreeva VA, Lemogne C, Hercberg S, Galan P, et al. Prospective association between adherence to the Mediterranean diet and risk of depressive symptoms in the French SU.VI.MAX cohort. Eur J Nutr. 2017;10:10.
Google Scholar
Lai JS, Oldmeadow C, Hure AJ, McEvoy M, Byles J, Attia J. Longitudinal diet quality is not associated with depressive symptoms in a cohort of middle-aged Australian women. Br J Nutr. 2016;115:842–50.
Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Serra Majem L, et al. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66:1090–8.
Sanchez-Villegas A, Henriquez-Sanchez P, Ruiz-Canela M, Lahortiga F, Molero P, Toledo E, et al. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015;13:197.
Tehrani AN, Salehpour A, Beyzai B, Farhadnejad H, Moloodi R, Hekmatdoost A, et al. Adherence to Mediterranean dietary pattern and depression, anxiety and stress among high-school female adolescents. Mediterr J Nutr Metab. 2018;11:73–83.
Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Shroff MR, Mason MA, Evans MK, et al. The sex-specific role of plasma folate in mediating the association of dietary quality with depressive symptoms. J Nutr. 2010;140:338–47.
Adjibade M, Lemogne C, Julia C, Hercberg S, Galan P, Assmann KE, et al. Prospective association between adherence to dietary recommendations and incident depressive symptoms in the French NutriNet-Sante cohort. Br J Nutr. 2018 Aug;120:290–300.
Loprinzi PD, Mahoney S. Concurrent occurrence of multiple positive lifestyle behaviors and depression among adults in the United States. J Affect Disord. 2014;165:126–30.
Rahmani J, Milajerdi A, Dorosty-Motlagh A. Association of the Alternative Healthy Eating Index (AHEI-2010) with depression, stress and anxiety among Iranian military personnel. J R Army Med Corps. 2017;15:15.
Saneei P, Hajishafiee M, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Adherence to Alternative Healthy Eating Index in relation to depression and anxiety in Iranian adults. Br J Nutr. 2016;116:335–42.
Akbaraly TN, Sabia S, Shipley MJ, Batty GD, Kivimaki M. Adherence to healthy dietary guidelines and future depressive symptoms: evidence for sex differentials in the Whitehall II study. Am J Clin Nutr. 2013;97:419–27.
Khayyatzadeh SS, Mehramiz M, Mirmousavi SJ, Mazidi M, Ziaee A, Kazemi-Bajestani SMR, et al. Adherence to a Dash-style diet in relation to depression and aggression in adolescent girls. Psychiatry Res. 2017;259:104–9.
Meegan AP, Perry IJ, Phillips CM. The association between dietary quality and dietary guideline adherence with mental health outcomes in adults: a cross-sectional analysis. Nutrients. 2017;9:05.
Perez-Cornago A, Sanchez-Villegas A, Bes-Rastrollo M, Gea A, Molero P, Lahortiga-Ramos F, et al. Relationship between adherence to Dietary Approaches to Stop Hypertension (DASH) diet indices and incidence of depression during up to 8 years of follow-up. Public Health Nutr. 2017;20:2383–92.
Valipour G, Esmaillzadeh A, Azadbakht L, Afshar H, Hassanzadeh A, Adibi P. Adherence to the DASH diet in relation to psychological profile of Iranian adults. Eur J Nutr. 2017;56:309–20.
Adjibade M, Andreeva VA, Lemogne C, Touvier M, Shivappa N, Hebert JR, et al. The inflammatory potential of the diet is associated with depressive symptoms in different subgroups of the general population. J Nutr. 2017;147:879–87.
Akbaraly T, Kerlau C, Wyart M, Chevallier N, Ndiaye L, Shivappa N, et al. Dietary inflammatory index and recurrence of depressive symptoms: results from the Whitehall II Study. Clin Psychol Sci. 2016;4:1125–34.
Bergmans RS, Malecki KM. The association of dietary inflammatory potential with depression and mental well-being among U.S. adults. Prev Med. 2017;99:313–9.
Phillips CM, Shivappa N, Hebert JR, Perry IJ. Dietary inflammatory index and mental health: a cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin Nutr. 2017;05:05.
Shivappa N, Hebert JR, Rashidkhani B. Association between inflammatory potential of diet and stress levels in adolescent women in Iran. Arch Iran Med. 2017;20:108–12.
PubMed Google Scholar
Shivappa N, Schoenaker DA, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women’s Health. Br J Nutr. 2016;116:1077–86.
Wirth MD, Shivappa N, Burch JB, Hurley TG, Hebert JR. The Dietary Inflammatory Index, shift work, and depression: results from NHANES. Health Psychol. 2017;36:760–9.
Sanchez-Villegas A, Ruiz-Canela M, De La Fuente-Arrillaga C, Gea A, Shivappa N, Hebert JR, et al. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr. 2015;114:1471–9.
Bloom I, Edwards M, Jameson KA, Syddall HE, Dennison E, Gale CR, et al. Influences on diet quality in older age: the importance of social factors. Age Ageing. 2017;46:277–83.
Collin C, Assmann KE, Andreeva VA, Lemogne C, Hercberg S, Galan P, et al. Adherence to dietary guidelines as a protective factor against chronic or recurrent depressive symptoms in the French SU.VI.MAX cohort. Prev Med. 2016;91:335–43.
Espana-Romero V, Artero EG, Lee DC, Sui X, Baruth M, Ruiz JR, et al. A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics. 2013;54:525–35.
Gall SL, Sanderson K, Smith KJ, Patton G, Dwyer T, Venn A. Bi-directional associations between healthy lifestyles and mood disorders in young adults: the childhood determinants of Adult Health Study. Psychol Med. 2016;46:2535–48.
Gomes AP, Oliveira Bierhals I, Goncalves Soares AL, Hellwig N, Tomasi E, Formoso Assuncao MC, et al. Interrelationship between diet quality and depressive symptoms in elderly. J Nutr Health Aging. 2017:1–6.
Huddy RL, Torres SJ, Milte CM, McNaughton SA, Teychenne M, Campbell KJ. Higher adherence to the Australian dietary guidelines is associated with better mental health status among Australian adult first-time mothers. J Acad Nutr Diet. 2016;116:1406–12.
Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med. 2011;73:483–90.
Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167:305–11.
Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS ONE [Electron Resour]. 2012;7:e52771.
Lai JS, Hure AJ, Oldmeadow C, McEvoy M, Byles J, Attia J. Prospective study on the association between diet quality and depression in mid-aged women over 9 years. Eur J Nutr. 2017;56:273–81.
Sakai H, Murakami K, Kobayashi S, Suga H, Sasaki S.Three-generation Study of Women on D, et al. Food-based diet quality score in relation to depressive symptoms in young and middle-aged Japanese women. Br J Nutr. 2017;117:1674–81.
Voortman T, Kiefte-de Jong JC, Ikram MA, Stricker BH, van Rooij FJA, Lahousse L, et al. . Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol. 2017;19:19.
Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608.
Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20.
Dixon LB, Subar AF, Peters U, Weissfeld JL, Bresalier RS, Risch A, et al. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr. 2007;137:2443–50.
Guenther ALB, Liese AD, Bell RA, Dabelea D, Lawrence JM, Rodriguez BL, et al. Association between the dietary approaches to hypertension diet and hypertension in youth with diabetes mellitus. Hypertension. 2009;53:6–12.
Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr.. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308–14.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96.
Anic GM, Park Y, Subar AF, Schap TE, Reedy J. Index-based dietary patterns and risk of lung cancer in the NIH-AARP diet and health study. Eur J Clin Nutr. 2016;70:123–9.
Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, et al. Associations of key diet-quality indexes with mortality in the multiethnic cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101:587–97.
Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, et al. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145:393–402.
Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanza-Martinez V, Freeman MP, et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271–4.
Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Med. 2015;13:215.
Article PubMed PubMed Central CAS Google Scholar
Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014;2014:563160.
Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–56.
Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14:207.
Sanchez-Villegas A, Martinez-Gonzalez MA. Diet, a new target to prevent depression? BMC Med. 2013;11:3.
Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223–44.
Kipnis V, Subar AF, Midthune D, Freedman LS, Ballard-Barbash R, Troiano RP, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158:14–21. discussion2–6
Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15:23.
Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci. 2017 Dec 7:1–14. https://doi.org/10.1080/1028415X.2017.1411320 . [Epub ahead of print]
Download references
MK is supported by the Medical Research Council (MRC K013351), NordForsk, and the Academy of Finland (311492). The funding bodies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Author contributions
CL and TA conducted the searches and data extraction. AB was responsible for resolving any discordance. CL and TA drafted the manuscript. CL conducted the statistical analysis. FJ, ASV, GDB, MK, and AB were responsible for interpreting the results and critically revising the manuscript. All authors have read and approved the final manuscript.
Author information
Authors and affiliations.
Department of Epidemiology and Public Health, University College London, London, WC1E 6BT, United Kingdom
Camille Lassale, G. David Batty, Mika Kivimäki & Tasnime Akbaraly
Department of Behavioural Science and Health, University College London, London, WC1E 6BT, United Kingdom
Camille Lassale
Department of Psychiatry & Autism Resources Centre, University Hospital of Montpellier, CHRU de Montpellier, F-34000, France
Amaria Baghdadli & Tasnime Akbaraly
INSERM, U1018, Centre for Research in Epidemiology and Population Health, Hôpital Paul Brousse, Villejuif, France
Amaria Baghdadli
Deakin University, Food & Mood Centre, IMPACT Strategic Research Centre, School of Medicine, Barwon Health, Geelong, Australia
Felice Jacka
Nutrition Research Group, Research Institute of Biomedical and Health Sciences, University of Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain
Almudena Sánchez-Villegas
Ciber de Fisiopatología de la Obesidad y Nutrición (CIBER OBN), Instituto de Salud Carlos III, Madrid, Spain
Clinicum, Faculty of Medicine, University of Helsinki, Helsinki, Finland
Mika Kivimäki
MMDN, University of Montpellier, EPHE, INSERM, U1198, Montpellier, F-34095, France
Tasnime Akbaraly
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Camille Lassale .
Ethics declarations
Conflict of interest.
Felice Jacka has received: (1) competitive Grant/Research support from the Brain and Behaviour Research Institute, the National Health and Medical Research Council (NHMRC), Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, The University of Melbourne; (2) industry support for research from Meat and Livestock Australia, Woolworths Limited, the A2 Milk Company, Be Fit Foods; (3) philanthropic support from the Fernwood Foundation, Wilson Foundation, the JTM Foundation, the Serp Hills Foundation, the Roberts Family Foundation, the Waterloo Foundation and; (4) travel support and speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Network Nutrition, Angelini Farmaceutica, Eli Lilly and Metagenics. Felice Jacka has written two books for commercial publication. All other authors declare that they have no conflict of interest.

Additional information
The original online version of this article was revised: Following publication of this article, Camille Lassale contacted the journal to request an addition to the ‘Conflict of Interest’ declaration.
Electronic supplementary material
Supplemental material, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lassale, C., Batty, G.D., Baghdadli, A. et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry 24 , 965–986 (2019). https://doi.org/10.1038/s41380-018-0237-8
Download citation
Received : 10 May 2018
Revised : 26 July 2018
Accepted : 02 August 2018
Published : 26 September 2018
Issue Date : July 2019
DOI : https://doi.org/10.1038/s41380-018-0237-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The association between dietary quality, sleep duration, and depression symptoms in the general population: findings from cross-sectional nhanes study.
- Xiaohong Ma
BMC Public Health (2024)
Factors associated with depressive symptoms among cancer patients: a nationwide cross-sectional study
- Xiaoqing Chen
Relationship of the Prime Diet Quality Score (PDQS) and Healthy Eating Index (HEI-2015) with depression and anxiety: a cross-sectional study
- Amirhossein Ataei Kachouei
- Farzam Kamrani
- Nizal Sarrafzadegan
A pragmatic randomized trial to examine the effect of combining healthy diet with mindfulness cognitive therapy to reduce depressive symptoms among university students in a low-resource setting: protocol for the NutriMind Project
- Kristin Reimers Kardel
- Per Ole Iversen
- Prudence Atukunda Friberg
BMC Psychiatry (2024)
Psychological distress and health behaviours in people living with and beyond cancer: a cross-sectional study
- Natalie Ella Miller
- Phillippa Lally
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Nutrition and mental health: A review of current knowledge about the impact of diet on mental health
Mateusz grajek, karolina krupa-kotara, agnieszka białek-dratwa, karolina sobczyk, martina grot, oskar kowalski, wiktoria staśkiewicz.
- Author information
- Article notes
- Copyright and License information
Edited by: Andrew Scholey, Swinburne University of Technology, Australia
Reviewed by: Monica Carrera, Spanish National Research Council (CSIC), Spain; Yu Xiao, Hunan Agricultural University, China
*Correspondence: Karolina Krupa-Kotara, [email protected]
This article was submitted to Nutrition, Psychology and Brain Health, a section of the journal Frontiers in Nutrition
Received 2022 May 14; Accepted 2022 Jul 20; Collection date 2022.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Applied psychopharmacotherapy and psychotherapy do not always bring the expected results in the treatment of mental disorders. As a result, other interventions are receiving increasing attention. In recent years, there has been a surge in research on the effects of nutrition on mental status, which may be an important aspect of the prevention of many mental disorders and, at the same time, may lead to a reduction in the proportion of people with mental disorders. This review aims to answer whether and to what extent lifestyle and related nutrition affect mental health and whether there is scientific evidence supporting a link between diet and mental health. A review of the scientific evidence was conducted based on the available literature by typing in phrases related to nutrition and mental health using the methodological tool of the PubMed database. The literature search yielded 3,473 records, from which 356 sources directly related to the topic of the study were selected, and then those with the highest scientific value were selected according to bibliometric impact factors. In the context of current changes, urbanization, globalization, including the food industry, and changes in people’s lifestyles and eating habits, the correlations between these phenomena and their impact on mental state become important. Knowledge of these correlations creates potential opportunities to implement new effective dietary, pharmacological, therapeutic, and above all preventive interventions. The highest therapeutic potential is seen in the rational diet, physical activity, use of psychobiotics, and consumption of antioxidants. Research also shows that there are nutritional interventions that have psychoprotective potential.
Keywords: nutrition, mental health, diet, psychology of food, eating behavior
Inherent in urbanization and the accompanying technological and cultural development, the rush of life, the pursuit of self-actualization, and the resulting overstimulation and lack of time, affect the change in eating habits and the consumption of high-calorie and processed foods ( 1 ). We can consider them as factors influencing the development of civilization diseases, important from the point of view of public health. Among them, we cannot forget about depressive and anxiety disorders that are becoming a global epidemic ( 2 ). The number of people requiring assistance from a mental health professional is steadily increasing in Poland and worldwide. According to the International Health Metrics Evaluation (IHME), at the end of 2017, 13% of the world population suffered from mental disorders ( 3 ). The Wittchen et al. study shows that mental disorders affect 38% of the European population ( 4 ). By the end of 2019, about 1.6 million people in Poland had received psychiatric treatment ( 5 ). The situation was not improved by the COVID-19 pandemic and related sanitary restrictions, which led to the isolation of many people, with feelings of insecurity, sadness, anxiety, and misinformation ( 6 ). All this has made psychological and psychiatric help the most sought-after form of health support today. There are only about 4,300 practicing psychiatrists in Poland ( 7 ). Even fewer, only 455, are practicing child psychiatry specialists ( 8 ). Statistics are believed to be better in the psychological and psychotherapeutic support sector, although public opinion is still divided about this form of support. Moreover, registers of psychologists and psychotherapists are not common. The described phenomena lead to a transformation of the psychiatric care model and mental health support. The number of people receiving psychiatric treatment is expected to increase over the next decades. The applied psychopharmacotherapy and psychotherapy do not always bring the expected treatment result ( 9 ). As a result, other interventions are receiving increasing attention. In recent years, there has been a dramatic increase in research on the effects of nutrition on mental status, which may be an important aspect of the prevention of many mental disorders, and at the same time may lead to a reduction in the proportion of people with mental disorders.
Thus, this review aims to answer the question of whether and to what extent lifestyle and related nutrition affect mental health and whether there is scientific evidence supporting the diet and mental health relationship.
The question posed in the objective can be divided into specific questions according to which this review was divided.
Are there correlations between nutrition and mental health?
Are there psychoprotective food ingredients?
Are there nutritional interventions with proven preventive potential for mental disorders?
Review methodology
Methodology background.
The main aspect that guided the review works conducted was to look for nutritional recommendations in the cited works regarding nutrition as psychoprophylaxis and dietary management of psychiatric disorders. Unfortunately, the current state of knowledge on this topic, despite many studies, is still poor, so the authors decided to conduct a broad review of the most current knowledge in this area to identify those sources that address the described topic and gather in one place the available knowledge.
Review procedure
The review was conducted following good practices associated with conducting similar reviews. Literature items were searched by a team of researchers (authors) along with a library staff member trained in literature searching and EBM (evidence-based medicine) and HTA (health technology assessment). A preliminary search for items consistent with the topic and purpose of the review was conducted to identify the research field. After reviewing existing data, a keyword package was selected that seemed most relevant and consistent with the review topic.
Eligibility criteria
The primary eligibility criteria were the language of publication, years of research or review, publication status, and whether the authors were specialists in their field (or had other publications in a similar field). Regarding language, English-language articles were selected because this language seems to be universal in the scientific community. In addition, articles that were published after 2005 were included to make sure that the topic addressed was not a completely new field of research, but also to avoid very old data, because as is known from common practice, dietetics, as well as mental health expertise, are two of the most rapidly developing scientific fields. Additionally, articles were selected that were available in full-text on an open-access basis and had impact factor values.
Search strategy
A review of the scientific evidence was conducted based on the available literature by entering sample phrases (consistent with the MeSh dictionary) with Boole operators, logical operators (and, or, not), and special characters,: “psychodietetics,” “nutripsychiatry,” “diet,” “mental health,” “lifestyle,” “body weight,” “obesity,” “depression,” “mental disorders” (and various combinations thereof) using the methodological tool of the PubMed database. The PubMed database in this regard seems most appropriate because it is a methodological tool that allows searching for articles available in multiple scientific databases (such as Medline or Embase). Its use provides the opportunity to meet all expectations from the review (transparency, clarity, comprehensiveness, focus, uniformity, accessibility, coverage of the entire topic).
Sources selection
The literature search yielded 3,473 records, from which 356 sources directly related to the topic of the study were selected, and then those with the highest scientific value were selected according to eligibility criteria.
The accuracy, objectivity, validity, and relevance of the evidence were tested using questions consistent with the GRADE scale: Is the information reliable? Is the information free of mistakes? Has the information been properly substantiated? Is it possible to verify the information against other reliable sources? Who are the authors? Are they qualified to present information on the topic? Are they affiliated with reputable institutions working on the issue? Is the data source peer-reviewed? For what purpose was the information? Is the information an evidenced-based fact or constitutes an opinion? Is the information subject to risk? Can this risk be estimated? When was the information published? Is the information current or outdated? Is the timeliness relevant to the issue at hand? Does the information cover the entire issue? Does the information contain background data or does it explore the issue in depth? The final literature review was based on 110 sources, representing mainly scientific output after 2005 and important multicenter studies performed after 2015. The data obtained from the review are presented in descriptive and tabular form. In addition, 11 additional sources were used in preparing the background of the research problem and the theoretical introduction.
Critical appraisal
In critically evaluating the sources, attention was paid to whether the articles appeared in peer-reviewed journals (by at least two reviewers) and whether they had an impact factor. As described above, 110 sources were eligible for final review. A limitation of the method adopted was primarily the exclusion of sources written in a language other than English. In addition, IF has many well-documented drawbacks as a research assessment tool and therefore is not the best way to evaluate the quality of individual research articles. Nevertheless, it was chosen because it is a synthetic indicator of a source’s impact on the field of science, and a journal that has it can more likely claim to be publishing credible scientific evidence. The review did not include so-called “grey literature”, i.e., literature that has not gone through the review process or that is internal to the university (theses, conference reports, government leaflets, newsletters, etc.). Despite their multiple values, these sources are characterized by a high risk of containing outdated knowledge ( Figure 1 ).
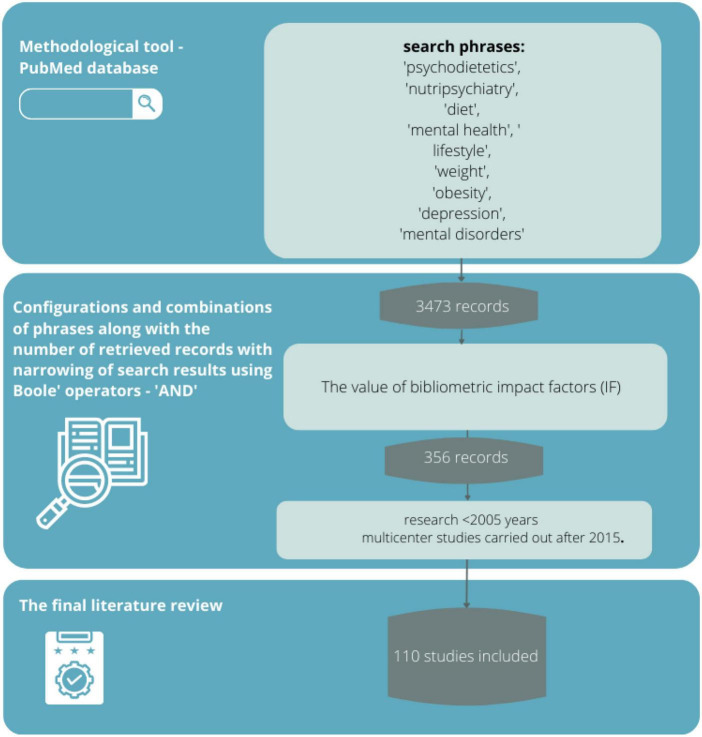
A flowchart of how to proceed in selecting bibliographic sources.
Q1: Are there correlations between nutrition and mental health?
Excess body weight is certainly an important social problem today. More than 0.7 billion people worldwide are obese, this is about 30% of the total population, and the number of obesity-related deaths is constantly increasing ( 10 ). We consume more and more processed, high-energy, and nutrient-poor foods. Consequently, we face problems of overweight and obesity with concomitant nutrient deficiencies (quantitative malnutrition) ( 11 ). Although the level of calories consumed is increasing, we are not taking in the recommended values of micro- and macroelements that play a significant role in the proper functioning of our nervous system – B vitamins, zinc, and magnesium. Additionally, we consume less fiber- and nutrient-rich vegetables and cereal products than recommended ( 10 , 11 ). Superimposing smoking, limited physical activity, and harmful alcohol consumption to the above dietary patterns, adversely affect health and development of mental disorders, including depression ( 10 ). Whose nutritional prevention is well documented in the literature ( 12 ).
The antioxidant system, which has been implicated in the development of psychiatric disorders, is relevant here ( 13 ) and its proper functioning depends on the presence of nutrients in food. In addition, the concentration of brain-derived neurotrophic factor (BDNF), which is involved in plasticity and neurodegenerative processes, depends on nutrients ( 14 ). Findings indicate a reduction in the incidence of depression and suicide with a healthy eating pattern ( 15 , 16 ). Randomized trials are emerging that evaluate the efficacy of dietary change as a form of treatment for depression ( 15 – 17 ). Selective food supplementation can be beneficial in the treatment of psychiatric disorders. Among them, compounds such as S-adenosylmethionine, N -acetylcysteine, zinc, and B vitamins including folic acid, and vitamin D are mentioned. Also, omega-3 unsaturated fatty acids have a wide range of effects. They participate in synaptogenesis by influencing receptor degradation and synthesis. They have an anti-inflammatory effect and inhibit apoptosis. They affect cell membrane function, BDNF action, and neurotransmitter reuptake ( 18 ). S-adenosylmethionine (SAM) is a compound formed from adenosine and methionine, which plays a key role in methylation processes. The results of studies show its antidepressant effects ( 19 ). The use of N -acetylcysteine influenced the effectiveness of therapy in schizophrenia, bipolar affective disorder, or trichotillomania. It has anti-inflammatory, antioxidant, and neuroprotective effects ( 20 ). Zinc deficiency, in turn, has been linked to the severity of depressive symptoms, and its supplementation included with antidepressants plays a role in mood stabilization. Zinc modulates cytokine activity and influences neurogenesis by affecting brain-derived neurotrophic factor levels ( 21 ). B vitamins play a role in the proper functioning of the nervous tissue. Folic acid (vitamin B9) deficiency has been associated with depressive symptoms and determined in subjects with mediocre responses to antidepressants ( 22 ). Low vitamin D levels were associated with a higher risk of schizophrenia and depression ( 23 ). It has been proven that vitamin D supplementation for a period of 3 months (4,000 IU/day for 1 month and 2,000 IU/day for 2 months) significantly reduced the severity of depression, irritability, fatigue, mood swings, sleep difficulties, weakness, and ability to concentrate in adolescents diagnosed with depression. This effect is supported by studies on animal models – vitamin D contributes to the plasticity of synapses, has a neuroprotective effect, supports the production of neurotrophic factors such as nerve growth factor (NGF) and regulates the function of the dopaminergic system. ( 24 ).
For the review, the results of the most important studies on the psychoprotective effects of bioactive components contained in foods (vitamins, minerals, omega-3, and more). have been collected in tabular form ( Table 1 ).
Review of selected studies on the psychoprotective effect of probiotics.
Source: Own compilation based on literature review.
Q2: Are there psychoprotective food ingredients?
The gut microbiota is estimated to form a complex ecosystem containing 1,014 microorganisms. It contains 3.3 million genes and outnumbers the human genome by about 150-fold. At the same time, it is built by more than a thousand different species of microorganisms ( 25 ). The gut-brain axis describing the bidirectional relationship between the gastrointestinal tract and the central nervous system uses several communication mechanisms. Mutual exchange of information can occur via the autonomic nervous system and the vagus nerve ( 26 ). Many of the effects of probiotics on mental status are associated with information transmission via the vagus nerve ( 27 ). Results from germ-free (GF) mice cultured under sterile conditions, devoid of detectable microorganisms, demonstrate the involvement of the gut microbiota in the proper formation and function of the endocrine system by influencing the development of the hypothalamic-pituitary-adrenal axis. The response to a stress stimulus as measured by glucocorticosteroid and adrenocorticotropin levels was significantly elevated in GF mice. It was normalized after gastrointestinal colonization with the Bifidobacterium infantis strain ( 28 ). Additionally, stress affects the formation and diversity of intestinal microflora ( 29 ). Another link of communication is the immune system. The microbiota is involved in the proper development of the gastrointestinal mucosal immune system ( 30 ). Bacterial antigens such as polysaccharide A, lipopolysaccharides, and thymic acids shape its proper functioning ( 31 ). The microbiota also produces neurotransmitters: gamma-aminobutyric acid, butyric acid, serotonin, dopamine, and short-chain fatty acids, which can directly affect the nervous system ( 32 ).
So, can the psychoprotective effect of strains be used in nutritional intervention? It seems reasonable here to consider the possibility of implementing treatment with probiotic preparations containing selected bacterial strains that show positive effects on the human psyche. In this approach, “probiotic” is defined as living organisms that, when consumed in adequate amounts, have a beneficial effect on the functioning of the body ( 33 ). Ilya Metchnikov was awarded the Nobel Prize in 1908 for his research on probiotics. Among them, lactic acid bacteria are the most popular. Probiotics are mainly found in fermented dairy products, or pickled products ( 34 ). Prebiotics are non-digested food components whose fermentation in the gastrointestinal tract stimulates either bacterial growth or activity or affects both, leading to the development of beneficial intestinal microflora ( 35 ). Prebiotics can include ingredients such as inulin or fructooligosaccharides. Prebiotics may also have a beneficial effect by inhibiting the growth of pathogenic bacteria. Moreover, some research results show that prebiotics can reduce inflammation by modifying the composition of the microbiota ( 36 ). Synbiotics are ingredients that contain both prebiotics and probiotics. Such a constellation allows the use of synergistic effects of these preparations. In turn, psychobiotics are defined as microorganisms that are probiotics, that show positive effects in patients treated for mental disorders ( 37 ). They can often achieve their effect through the production of neurotransmitters such as gamma-aminobutyric acid, serotonin, or other substances with an effect on the cells of the nervous system such as short-chain organic acids: acetic, propionic, or butyric ( 36 ). Oral substitution of such probiotics as Lactobacillus helveticus and Bifidobacterium longum over a period of 1 month was associated with a reduction in symptoms of anxiety and depressive disorders and a reduction in stress levels as measured by the determination of cortisol levels in animal models ( 38 ). Currently, the most effective treatment of psychiatric disorders is achieved through the use of antidepressants, or antipsychotics. However, the additional use of psychobiotics to treat anxiety or depressive disorders may prove effective in the future. It is also worth noting that popular antidepressants and antipsychotics can affect the quality of gut flora and change the composition of the microbiome to a disadvantage by killing the cultures of bacteria living in the gastrointestinal tract ( 39 ).
For the review, the results of the most important studies on the psychoprotective effect of probiotics were collected in tabular form – Table 2 .
Review of selected studies on the psychoprotective effects of substances contained in food.
Factors such as genotype, intrauterine infections, developmental disorders, later traumatizing events, use of harmful psychoactive substances, and many others will influence the onset of psychiatric disorders. These factors influence not only the onset of the disorder but also its progression. Treating early conditions in psychiatry can result in a much better response to the treatment given and better functioning of patients. This fact can be particularly observed in studies on the early detection of psychotic disorders ( 40 ). Prevention in medicine, including psychiatry, requires knowledge of appropriate and useful tools that would allow detection of increased risk of mental illness and monitoring of the developing psychopathology of the disorder. McGorry et al. ( 41 ) proposed a four-stage model of the development of mental disorders. According to this model, serious mental disorders develop from high-risk states: grade 0 means the development of undifferentiated, general symptoms, such as slight anxiety, restlessness, depressive symptoms, or somatic symptoms lead to grade 1, in which types 1A and 1B can be distinguished according to their severity. Further progression of the disease results in the development of a first episode of the disorder and here we speak of stage 2, which is accompanied by persistent 7ncludims and frequent relapses. Grade 3 includes incomplete remission and regular and repeated relapses. Grade 4 in this context means treatment-resistant disorder. The worsening of a psychiatric disorder is determined by genetic and environmental factors, and it is the latter that seems to be the main target for preventive interventions in psychiatry. Some biomarkers in psychiatry are directly related to nutrition. The first of these is the hypothalamic-pituitary-adrenal axis (HPA). Reduced ability to cope with stress plays a role in the development of psychiatric disorders ( 42 ). It is known that traumatizing experiences in early childhood shape vulnerability to stress in later life ( 43 ). The normal functioning of the HPA axis is often altered in psychiatric disorders, and increased cortisol secretion is observed in affective and psychotic disorders. Additionally, antipsychotic drugs appear to decrease HPA axis activity ( 44 – 47 ). Furthermore, healthy individuals who were first-degree relatives of individuals with psychotic disorders were found to have HPA axis dysfunction with elevated cortisol levels ( 48 ). These studies show that the HPA axis appears to be an important biological marker of susceptibility to developing psychiatric disorders. In this context, its association with gut microbiota is not insignificant. Other potential biomarkers involved in the pathophysiology of psychiatric disorders are inflammation and oxidative stress ( 49 ). The inflammatory theory of depression development is gaining increasing attention, and elevated levels of proinflammatory cytokines are observed in depressive, psychotic, and manic states ( 50 , 51 ). Elevated levels of proinflammatory cytokines occur before the onset of de novo disorders, suggesting their role in the genesis of these disorders ( 52 ). An increase in oxidative stress in psychotic disorders with a decrease in glutathione and antioxidant enzymes has also been observed ( 53 ). The potential effectiveness of selective cyclooxygenase-2 antagonists in the treatment of bipolar affective disorder and schizophrenia has been demonstrated ( 51 , 54 ). The use of statins, which have anti-inflammatory and antioxidant properties, reduced the risk of depressive disorders ( 55 ). Polyunsaturated fatty acids are further potential biomarkers that may have applications in psychiatry. Omega-3 polyunsaturated fatty acids may play a role in the pathogenesis of affective and psychotic disorders ( 56 , 57 ). Their deficiency may be present in the early stages of psychotic disorders – stage 1b. Supplementation with omega-3 polyunsaturated fatty acids reduced the risk of psychotic disorders among individuals at high risk of developing them ( 58 ).
The intestinal barrier is composed of several layers, including the intestinal microflora, mucus layer, intestinal epithelium, and elements of the circulatory, immune, nervous, and lymphatic systems. The layer of epithelial cells, mainly enterocytes connected by tight junctions, is the most important for the intestinal barrier ( 59 ). Its main function is to regulate the absorption of nutrients, electrolytes, and water from the gastrointestinal lumen into the blood or lymphatic system and prevent the penetration of pathogens from the gastrointestinal lumen. Factors such as stress, pro-inflammatory factors, dysbacteriosis of the intestinal microflora, alcohol, or antibiotics may cause excessive permeability of the intestinal barrier ( 60 – 62 ). Currently, the microbiota and its diversity as a trigger for generalized inflammation are gaining great importance ( 61 ) Under the influence of the impaired functioning of the barrier, the migration of bacteria from the lumen of the gastrointestinal tract occurs, which activates the cells of the immune system affecting the functioning of the immune, endocrine and nervous systems ( 62 ). It has been observed that patients with depression have elevated IgA and IgM immunoglobulins against lipopolysaccharides of the bacterial microbiome ( 63 ). The current study indicates the use of a dietary inflammatory index, which assesses the effect of the entire diet or individual dietary components on the concentration of inflammatory markers. The results of a systematic review by Chen et al. ( 64 ) indicate that a higher dietary inflammatory index is associated with an increased risk of common psychiatric disorders, including symptoms of depression, anxiety, distress, and schizophrenia. Of particular importance is the novel finding from the dose-response analysis that a 1 unit increase in the dietary inflammatory index was associated with a 6% higher risk of depressive symptoms. Similar relationships have been observed by Firth et al. ( 63 ), particularly in schizophrenia – individuals who consume more pro-inflammatory foods and less anti-inflammatory foods are more predisposed to psychiatric disorders. At this point, it is important to look at the relationship between diet and the proper functioning of the intestinal barrier. It turns out that it is not without significance in maintaining homeostasis. A diet consisting of fast food and highly processed foods is associated with increased intestinal barrier permeability ( 65 , 66 ).
Q3: Are there nutritional interventions with proven preventive potential for mental disorders?
Epidemiological studies have shown that diet impacts mental health, and intervention studies confirm this relationship ( 17 ). The challenge for “nutritional psychiatry” is to produce comprehensive, consistent, and scientifically rigorous evidence-based studies that define the role of diet and nutrients in different aspects of mental health ( 67 – 70 ). Overall, few randomized trials investigate the effectiveness of dietary change in mental health treatment. One intervention study to date involved a 12-week Mediterranean diet. This study reported significant improvements in mood and reduced anxiety in adults with major depression ( 71 ) More recent RCTs – HELFIMED ( 72 ) and PREDI_DEP ( 73 ) have confirmed the benefits of a Mediterranean-style diet for mental health in depression. In contrast to these studies, in the MooDFOOD RCT, multiple nutrient supplementation did not reduce episodes of major depression in overweight or obese adults with subsyndromal depressive symptoms. This study found that multinutrient supplements containing omega-3 PUFAs, vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility indices ( 74 ). Similar results regarding the lack of effect on mental state improvement were obtained in a review of the literature in the context of vitamin D ( 75 ). For omega-3 PACs, one RCT including people with mild to moderate depression found no beneficial effect of omega-3 PACs on depressive symptoms ( 76 ). No effect of folic acid supplementation in combination with vitamin B 6 and B 12 on the onset of depression was found in older men ( 77 ) and older women ( 78 ). Furthermore, Rayman et al. ( 79 ) found no effect of selenium supplementation on mood in older people. Overall, the studies available to date, do not support the use of nutritional supplementation to prevent depression.
However, many studies confirm that higher dietary quality in adulthood is associated with a reduced risk of cognitive decline ( 17 ). Additionally, the intake of antioxidant polyphenols in older adults is associated with improved cognitive ability ( 80 – 82 ). Another study showed that a Mediterranean diet supplemented with olive oil and nuts was associated with improved cognitive function in an older population ( 83 ).
Therefore, we undertook an analysis of diets that could potentially affect mental health such as the MIND diet, the Mediterranean diet, and the ketogenic diet.
The MIND diet is a dietary recommendation to counteract neurodegenerative brain changes and improve nervous system function. This diet is beneficial for cognitive decline in the aging process, as well as for the prevention and progression of neurodegenerative diseases, including Alzheimer’s disease ( 84 ). The MIND diet combines the principles of the Mediterranean diet and the DASH diet, which are based on a high intake of vegetables, fruits, nuts, whole grain cereal products, olive oil, fish, and seafood, and moderate consumption of dry red wine with meals ( 85 ). Studies prove the positive effects of the DASH and Mediterranean diets on other diseases such as diabetes, cancer, and obesity ( 86 – 89 ).
Long-term observations confirm that adherence to the Mediterranean diet reduces the risk of developing neurological disorders by up to 28% compared to the use of other diets ( 83 ). Adherence to the MIND diet was significantly associated with a lower chance of depression and psychological distress, but not anxiety, in the entire study population ( 90 ). Like the Mediterranean diet and the DASH diet, the MIND diet emphasizes natural plant-based foods and limited intake of animal and high-fat foods, especially of animal origin. However, there are some differences between the MIND diet and the DASH diet, and the Mediterranean diet. For example, leafy green vegetables and especially berries are unique components of the MIND diet that are not included in the Mediterranean and DASH diets ( 90 ). The MIND diet does not focus on a high intake of fruit, dairy products, and potatoes. Another difference between MIND and the DASH and Mediterranean diets concerns fish consumption. In MIND, individuals consuming as little as 1 portion of fish per week receive a positive result, whereas, in the Mediterranean and DASH diets, larger amounts of fish would need to be consumed to achieve a result ( 91 ). The MIND diet significantly slows cognitive decline with age ( 92 ). The Mediterranean diet has also been shown to have a protective effect on anxiety and mental stress ( 93 ).
Mental illnesses are associated with numerous metabolic disorders in the brain and co-occur with many other metabolic disorders such as obesity, diabetes, and CVD. The ketogenic diet is an evidence-based treatment for epilepsy that has been shown to have profound effects on brain metabolism and neurotransmitter function. In a ketogenic diet, as much as 80 percent of energy can come from fat. This proportion sounds like a deal-breaker for healthy eating, but it turns out that ketones formed from fats can alleviate epileptic seizures unresponsive to anticonvulsant drug therapy ( 83 ). In the case of mitochondrial epilepsy, reports on the effects of the ketogenic diet are conflicting. In a study by El Sabbagh et al. ( 94 ), no patients on a ketogenic diet achieved no significant reduction in seizure frequency epileptic seizures. In contrast, a study by Kang et al. ( 95 ) involving 14 patients showed that the use of a ketogenic diet in 10 of them reduced the frequency of epileptic seizures by more than 50%, and in 7 patients, epileptic seizures ceased. In the analysis, there were improvements in symptoms including mood, cognitive function, communication skills, energy, anxiety, and auditory and visual hallucinations ( 90 ). Other reported benefits included positive biometric changes such as improvements in lipid profile, weight reduction, positive change in blood glucose, and reduction in HbA1c. These benefits may facilitate the management of comorbidities and improve overall health and well-being ( 93 ). This highlights that advances in nutritional psychiatry are important and it will be important to replicate, refine and scale up dietary intervention studies targeting the prevention and treatment of common mental health disorders. In addition, there is an unmet need for more randomized, controlled clinical trials ( 118 – 121 ).
Strengths and limitations
There is still little work in the scientific space that summarizes the major findings related to the impact of nutrition on mental health, especially, as this review does, highlighting the importance of nutrition in psychoprevention and pointing to the psychoprotective effects of nutrients. The primary limitation of the presented review of research on the relationship between diet and mental health is the plethora of studies on the topic. The plethora of studies here does not mean that they all address the issue presented in this manuscript. Much of the work that was searched and queried assumes a relationship between nutrition and the psyche, but these tend to be very superficial opinions that are not scientifically grounded. The authors are aware that in the face of such a large body of research, important reports may have been overlooked, but it should be noted that every effort was made to ensure that this review was conducted fairly, taking into account large, multi-center research projects and highlighting the major research streams in psychodietetics and nutripsychiatry.
Additionally, it was observed that in the current state of scientific knowledge, few large meta-analyses are treating the effects of food and diet on mental health. Therefore, it is difficult to discuss the effectiveness of introducing nutritional interventions among people with mental disorders or treating nutrition as the only means of prevention. Furthermore, the primary threat to interventions of this type is the difficulty in monitoring dietary patterns or intake of specific components. In addition, their absorption and metabolism are also dependent on many factors that rarely have a consistent course. Therefore, it is postulated that further research should be directed toward the creation of unambiguous dietary recommendations for mental health problems.
In recent decades, the relationship between nutrition and patients’ mental status has been underappreciated, as evidenced by the lack of research conducted before the 21st century in this area of knowledge – cited in this review. In recent years, this trend has been reversed, with research in psychodietetics and nutripsychiatry gaining popularity. In the context of current changes, urbanization, globalization, including the food industry, and changes in people’s lifestyles and eating habits, correlations between these phenomena and their impact on psychological status are becoming important. Exploring these correlations creates potential opportunities to implement new effective dietary, pharmacological, therapeutic, and above all preventive interventions ( Figure 2 ).

Links between nutrition and mental health.
Author contributions
MATG: conceptualization. MATG and KK-K: investigation and methodology. KS and AB-D: data curation. MATG: writing – original draft preparation. MATG, KK-K, MARG, and AB-D: writing – review and editing. KS and AB-D: supervision. KK-K: project administration. WS: conducting an additional literature review, creating tables summarizing current knowledge of psychobiotics and psychoprotective food ingredients, and revising the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- 1. Hidaka BH. Depression as a disease of modernity: Explanations for increasing prevalence. J Affect Disord. (2012) 140:205–14. 10.1016/j.jad.2011.12.036 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Baxter AJ, Patton G, Scott KM, Degenhardt L, Whiteford HA. Global epidemiology of mental disorders: what are we missing? PLoS One. (2013) 8:e65514. 10.1371/journal.pone.0065514 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. IHME. Data visualizations. Seattle, WA: IHME; (2020) [ Google Scholar ]
- 4. Wittchen HU, Fuetsch M, Sonntag H, Müller N, Liebowitz M. Disability and quality of life in pure and comorbid social phobia. Findings from a controlled study. Eur Psychiatry. (2000) 15:46–58. 10.1016/s0924-9338(00)00211-x [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. NPOZP. Narodowy program ochrony zdrowia psychicznego na lata 2017-2022. Rozporządzenie rady ministrów z dnia 8 lutego 2017 roku. Warsaw: Ministerstwo Zdrowia; (2017) [ Google Scholar ]
- 6. Hawes MT, Szenczy AK, Klein DN, Hajcak G, Nelson BD. Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol Med. (2021) 5:1–9. 10.1017/S0033291720005358 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Notes From Poland. Stigmatisation and medication: Poland’s outdated approach to mental health. New York, NY: NFP; (2020). [ Google Scholar ]
- 8. Pawłowski T, Kiejna A. Pathways to psychiatric care and reform of the public health care system in Poland. Eur Psychiatry. (2004) 19:168–71. 10.1016/j.eurpsy.2003.09.009 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. (2008) 5:e45. 10.1371/journal.pmed.0050045 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Parker E, Goldman J, Moshfegh A. America’s nutrition report card: comparing WWEIA, NHANES 2007-2010 Usual nu-trient intakes to dietary reference intakes. FASEB J. (2014) 28:384.2. [ Google Scholar ]
- 11. Opie RS, Itsiopoulos C, Parletta N, Sanchez-Villegas A, Akbaraly TN, Ruusunen A, et al. Dietary recommendations for the prevention of depression. Nutr Neurosci. (2017) 20:161–71. 10.1179/1476830515Y.0000000043 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. (2013) 34:167–77. 10.1016/j.tips.2013.01.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Diniz BS, Mendes-Silva AP, Silva LB, Bertola L, Vieira MC, Ferreira JD, et al. Oxidative stress markers imbalance in late-life depression. J Psychiatr Res. (2018) 102:29–33. 10.1016/j.jpsychires.2018.02.023 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Guimarães LR, Jacka FN, Gama CS, Berk M, Leitão-Azevedo CL, Belmonte de Abreu MG, et al. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1595–8. 10.1016/j.pnpbp.2008.06.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99:181–97. 10.3945/ajcn.113.069880 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: Shared pathology and treatment? Br. J. Clin. Pharmacol . (2011) 71:365–76. 10.1111/j.1365-2125.2010.03830.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the “SMILES” trial). BMC Med. (2017) 15:23. 10.1186/s12916-017-0791-y [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Mischoulon D, Freeman MP. Omega-3 fatty acids in psychiatry. Psychiatr Clin North Am. (2013) 36:15–23. 10.1016/j.psc.2012.12.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Sarris J, Papakostas GI, Vitolo O, Fava M, Mischoulon D. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. (2014) 164:76–81. 10.1016/j.jad.2014.03.041 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan A, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. (2013) 11:200. 10.1186/1741-7015-11-200 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Lai J, Moxey A, Nowak G, Vashum K, Bailey K, McEvoy M. The efficacy of zinc supplementation in depression: systematic review of randomised controlled trials. J Affect Disord. (2012) 136:e31–9. 10.1016/j.jad.2011.06.022 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Fava M, Mischoulon D. Folate in depression: efficacy, safety, differences in formulations, and clinical issues. J Clin Psychiatry. (2009) 70:12–7. 10.4088/JCP.8157su1c.03 [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. (2013) 34:47–64. 10.1016/j.yfrne.2012.07.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Brouwer-Brolsma EM, Dhonukshe-Rutten RA, van Wijngaarden JP, van der Zwaluw NL, Sohl E, In’t Veld PH, et al. Low vitamin D status is associated with more depressive symptoms in dutch older adults. Eur J Nutr. (2016) 55:1525–34. 10.1007/s00394-015-0970-6 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. 10.1038/nature11550 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. (2004) 16:55–9. 10.1111/j.1743-3150.2004.00476.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. (2014) 817:115–33. 10.1007/978-1-4939-0897-4_5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalam-ic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. 10.1113/jphysiol.2004.063388 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Bangsgaard Bendtsen KM, Krych L, Sørensen DB, Pang W, Nielsen DS, Josefsen K, et al. Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One. (2012) 7:e46231. 10.1371/journal.pone.0046231 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Mayer L. Mucosal immunity. Pediatrics. (2003) 111:1595–600. [ PubMed ] [ Google Scholar ]
- 31. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118:229–41. 10.1016/j.cell.2004.07.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Lyte M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. (2014) 5:381–9. 10.4161/gmic.28682 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Grot M, Krupa-Kotara K, Wypych-Ślusarska A, Grajek M, Białek-Dratwa A. The concept of intrauterine programming and the development of the neonatal microbiome in the prevention of SARS-CoV-2 infection. Nutrients. (2022) 14:1702. 10.3390/nu14091702 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Mackowiak PA. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. (2013) 1:52. 10.3389/fpubh.2013.00052 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Durchschein F, Petritsch W, Hammer HF. Diet therapy for inflammatory bowel diseases: The established and the new. World J Gastroenterol. (2016) 22:2179–94. 10.3748/wjg.v22.i7.2179 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. (2015) 12:303–10. 10.1038/nrgastro.2015.47 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, et al. Assessment of psychotropic-like properties of a probiotic formulation ( Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. (2011) 105:755–64. 10.1017/S0007114510004319 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation ( Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. (2011) 2:256–61. 10.4161/gmic.2.4.16108 [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Cussotto S, Clarke G, Dinan TG, Cryan JF. Psychotropics and the microbiome: A chamber of secrets. Psychopharmacology. (2019) 236:1411–32. 10.1007/s00213-019-5185-8 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Hegelstad WT, Larsen TK, Auestad B, Evensen J, Haahr U, Joa I, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. (2012) 169:374–80. 10.1176/appi.ajp.2011.11030459 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. (2010) 55:486–97. 10.1177/070674371005500803 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, Wood SJ, et al. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. (2006) 40:725–41. 10.1080/j.1440-1614.2006.01877.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology. (2013) 38:111–23. 10.1038/npp.2012.149 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. (2016) 63:327–42. 10.1016/j.psyneuen.2015.10.014 [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, et al. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. (2005) 30:1923–31. 10.1038/sj.npp.1300766 [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Cohrs S, Röher C, Jordan W, Meier A, Huether G, Wuttke W, et al. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology. (2006) 185:11–8. 10.1007/s00213-005-0279-x [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. (2011) 41:2305–15. 10.1017/S0033291711000602 [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. (2011) 70:663–71. 10.1016/j.biopsych.2011.04.013 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. (2011) 24:519–25. 10.1097/YCO.0b013e32834b9db6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Wadee AA, Kuschke RH, Wood LA, Berk M, Ichim L, Maes M. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. (2002) 17:175–9. 10.1002/hup.390 [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Clinical implications of the cytokine hy-pothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. (2010) 79:323–5. 10.1159/000319530 [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. (2009) 19:220–30. 10.1016/j.conb.2009.05.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, et al. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psycho-Pharmacol. (2008) 23:87–94. 10.1002/hup.912 [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. (2010) 121:118–24. 10.1016/j.schres.2010.04.015 [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. (2006) 163:969–78. 10.1176/ajp.2006.163.6.969 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. (1998) 30:193–208. 10.1016/s0920-9964(97)00151-5 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. (2010) 67:146–54. 10.1001/archgenpsychiatry.2009.192 [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. (2009) 87:101–8. 10.2527/jas.2008-1339 [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, et al. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. (1999) 112:137–46. 10.1242/jcs.112.1.137 [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. (2014) 111:E4485–93. 10.1073/pnas.1415174111 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Tulstrup MV, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O, et al. Antibiotic treatment affects intestinal permeability and gut microbial composition in wistar rats dependent on antibiotic class. PLoS One. (2015) 10:e0144854. 10.1371/journal.pone.0144854 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. (2008) 29:117–24. [ PubMed ] [ Google Scholar ]
- 63. Firth J, Veronese N, Cotter J, Shivappa N, Hebert JR, Ee C, et al. What is the role of dietary inflammation in severe mental illness? A review of observational and experimental findings. Front Psychiatry. (2019) 10:350. 10.3389/fpsyt.2019.00350 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Chen GQ, Peng CL, Lian Y, Wang BW, Chen PY, Wang GP. Association between dietary inflammatory index and mental health: A systematic review and dose-response meta-analysis. Front Nutr. (2021) 5:662357. 10.3389/fnut.2021.662357 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Sánchez-Villegas A, Toledo E, de Irala J, Ruiz-Canela M, Pla-Vidal J, Martínez-González MA. Fast-food and commercial baked goods consumption and the risk of depression. Public Health Nutr. (2012) 15:424–32. 10.1017/S1368980011001856 [ DOI ] [ PubMed ] [ Google Scholar ]
- 66. Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, et al. Nutritional psychiatry: Towards im-proving mental health by what you eat. Eur Neuropsychopharmacol. (2019) 29:1321–32. 10.1016/j.euroneuro.2019.10.011 [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. (2017) 76:427–36. 10.1017/S0029665117002026 [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, et al. Nutritional medicine as main-stream in psychiatry. Lancet Psychiatry. (2015) 2:271–4. 10.1016/S2215-0366(14)00051-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, et al. International society for nutri-tional psychiatry research consensus position statement: nutritional medicine in modern psychiatry. World Psychiatry. (2015) 14:370–1. 10.1002/wps.20223 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Jacka FN, O’Neil A, Itsiopoulos C, Opie R, Mohebbi M, Castle D, et al. The SMILES trial: An important first step. BMC Med. (2018) 16:237. 10.1186/s12916-018-1228-y [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr Neurosci. (2019) 22:474–87. 10.1080/1028415X.2017.1411320 [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Sánchez-Villegas A, Cabrera-Suárez B, Molero P, González-Pinto A, Chiclana-Actis C, Cabrera C, et al. Preventing the recurrence of depression with a Mediterranean diet supplemented with extra-virgin olive oil. The PREDI-DEP trial: study protocol. BMC Psychiatry. (2019) 19:63. 10.1186/s12888-019-2036-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 73. Berk M, Jacka FN. Diet and depression-from confirmation to implementation. JAMA. (2019) 321:842–3. 10.1001/jama.2019.0273 [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Bot M, Brouwer IA, Roca M, Kohls E, Penninx BWJH, Watkins E, et al. Effect of multinutrient supplementation and food-related behavioral activation therapy on prevention of major depressive disorder among overweight or obese adults with subsyndromal depressive symptoms: The MooDFOOD randomized clinical trial. JAMA. (2019) 321:858–68. 10.1001/jama.2019.0556 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. J Affect Disord. (2016) 198:1–14. 10.1016/j.jad.2016.03.022 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Ford AH, Flicker L, Thomas J, Norman P, Jamrozik K, Almeida OP. Vitamins B12, B6, and folic acid for onset of depressive symptoms in older men: results from a 2-year placebo-controlled randomized trial. J Clin Psychiatry. (2008) 69:1203–9. 10.4088/JCP.v69n0801 [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Okereke OI, Cook NR, Albert CM, Van Denburgh M, Buring JE, Manson JE. Effect of long-term supplementation with folic acid and B vitamins on risk of depression in older women. Br J Psychiatry. (2015) 206:324–31. 10.1192/bjp.bp.114.148361 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, Quintana M, Corella D, Pintó X, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. (2012) 29:773–82. 10.3233/JAD-2012-111799 [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Rayman M, Thompson A, Warren-Perry M, Galassini R, Catterick J, Hall E, et al. Impact of selenium on mood and quality of life: a randomized, controlled trial. Biol Psychiatry. (2006) 59:147–54. 10.1016/j.biopsych.2005.06.019 [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. (2014) 34:7862–70. 10.1523/JNEUROSCI.0385-14.2014 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Anton SD, Embry C, Marsiske M, Lu X, Doss H, Leeuwenburgh C, et al. Safety and metabolic outcomes of resveratrol supplementation in older adults: results of a twelve-week, placebo-controlled pilot study. Exp Gerontol. (2014) 57:181–7. 10.1016/j.exger.2014.05.015 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, et al. Mediterranean Diet and age-related cognitive decline: A randomized clinical trial. JAMA Intern Med. (2015) 175:1094–103. 10.1001/jamainternmed.2015.1668 [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. (2015) 11:1015–22. 10.1016/j.jalz.2015.04.011 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Dutta R. Role of MIND diet in preventing dementia and Alzheimer’s disease. ARC J Neurosci. (2019) 4:1–8. 10.20431/2456-057X.0403001 [ DOI ] [ Google Scholar ]
- 85. Said MS, El Sayed IT, Ibrahim EE, Khafagy GM. Effect of DASH Diet Versus healthy dietary advice on the estimated atherosclerotic cardiovascular disease risk. J Prim Care Community Health. (2021) 12:2150132720980952. 10.1177/2150132720980952 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. (2019) 11:338. 10.3390/nu11020338 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. (2019) 124:779–98. 10.1161/CIRCRESAHA.118.313348 [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Salas-Salvadó J, Becerra-Tomás N, García-Gavilán JF, Bulló M, Barrubés L. Mediterranean diet and cardiovascular disease prevention: What do we know? Prog Cardiovasc Dis. (2018) 61:62–7. 10.1016/j.pcad.2018.04.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 89. Salari-Moghaddam A, Keshteli AH, Mousavi SM, Afshar H, Esmaillzadeh A, Adibi P. Adherence to the MIND diet and prevalence of psychological disorders in adults. J Affect Disord. (2019) 256:96–102. 10.1016/j.jad.2019.05.056 [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Hodge A, Almeida OP, English DR, Giles GG, Flicker L. Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. Int Psychogeriatr. (2013) 25:456–66. 10.1017/S1041610212001986 [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Olveira C, Olveira G, Espildora F, Girón RM, Vendrell M, Dorado A, et al. Mediterranean diet is associated on symptoms of depression and anxiety in patients with bronchiectasis. Gen Hosp Psychiatry. (2014) 36:277–83. 10.1016/j.genhosppsych.2014.01.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Norwitz NG, Dalai SS, Palmer CM. Ketogenic diet as a metabolic treatment for mental illness. Curr Opin Endocrinol Diabetes Obes. (2020) 27:269–74. 10.1097/MED.0000000000000564 [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Tillery EE, Ellis KD, Threatt TB, Reyes HA, Plummer CS, Barney LR. The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin. (2021) 11:211–9. 10.9740/mhc.2021.05.211 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. El Sabbagh S, Lebre AS, Bahi-Buisson N, Delonlay P, Soufflet C, Boddaert N, et al. Epileptic phenotypes in children with respiratory chain disorders. Epilepsia. (2010) 51:1225–35. 10.1111/j.1528-1167.2009.02504.x [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Kang HC, Lee YM, Kim HD. Mitochondrial disease and epilepsy. Brain Dev. (2013) 35:757–61. 10.1016/j.braindev.2013.01.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Gazerani P. Migraine and diet. Nutrients. (2020) 12:1658. 10.3390/nu12061658 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Cater RJ, Chua GL, Erramilli SK, Keener JE, Choy BC, Tokarz P, et al. Structural basis of omega-3 fatty acid transport across the blood-brain barrier. Nature. (2021) 595:315–9. 10.1038/s41586-021-03650-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN. Dietary flaxseed as a strategy for improving human health. Nutrients. (2019) 11:1171. 10.3390/nu11051171 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Park M, Choi J, Lee HJ. Flavonoid-rich orange juice intake and altered gut microbiome in young adults with depressive symptom: A randomized controlled study. Nutrients. (2020) 12:1815. 10.3390/nu12061815 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Mulati A, Ma S, Zhang H, Ren B, Zhao B, Wang L, et al. Sea-buckthorn flavonoids alleviate high-fat and high-fructose diet-induced cognitive impairment by inhibiting insulin resistance and neuroinflammation. J Agric Food Chem. (2020) 68:5835–46. 10.1021/acs.jafc.0c00876 [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol. (2017) 232:2359–72. 10.1002/jcp.25518 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Fernández MJF, Valero-Cases E, Rincon-Frutos L. Food components with the potential to be used in the therapeutic approach of mental diseases. Curr Pharm Biotechnol. (2019) 20:100–13. 10.2174/1389201019666180925120657 [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Dogan-Sander E, Mergl R, Willenberg A, Baber R, Wirkner K, Riedel-Heller SG, et al. Inflammation and the association of vitamin D and depressive symptomatology. Nutrients. (2021) 13:1972. 10.3390/nu13061972 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Godos J, Currenti W, Angelino D, Mena P, Castellano S, Caraci F, et al. Diet and Mental health: review of the recent updates on molecular mechanisms. Antioxidants Basel. (2020) 9:346. 10.3390/antiox9040346 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Burton-Freeman BM, Sandhu AK, Edirisinghe I. Red raspberries and their bioactive polyphenols: cardiometabolic and neuronal health links. Adv Nutr. (2016) 7:44–65. 10.3945/an.115.009639 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res. (2008) 28:1–5. 10.1016/j.nutres.2007.10.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Wallace CJK, Foster JA, Soares CN, Milev RV. The effects of probiotics on symptoms of depression: protocol for a Dou-ble-blind randomized placebo-controlled trial. Neuropsychobiology. (2020) 79:108–16. 10.1159/000496406 [ DOI ] [ PubMed ] [ Google Scholar ]
- 108. Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr. (2019) 38:522–8. 10.1016/j.clnu.2018.04.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus plantarum 299v de-creases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. (2019) 100:213–22. 10.1016/j.psyneuen.2018.10.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Heidarzadeh-Rad N, Gökmen-Özel H, Kazemi A, Almasi N, Djafarian K. Effects of a psychobiotic supplement on serum brain-derived neurotrophic factor levels in depressive patients: A post hoc analysis of a randomized clinical trial. J Neurogastroenterol Motil. (2020) 26:486–95. 10.5056/jnm20079 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, et al. Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol. (2018) 15:662. 10.3389/fneur.2018.00662 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front Aging Neurosci. (2016) 10:256. 10.3389/fnagi.2016.00256 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 113. Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin Nutr. (2019) 38:2569–75. 10.1016/j.clnu.2018.11.034 [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Wallis A, Ball M, Butt H, Lewis DP, McKechnie S, Paull P, et al. Open-label pilot for treatment targeting gut dysbiosis in myalgic encephalomyelitis/chronic fatigue syndrome: neuropsychological symptoms and sex comparisons. J Transl Med. (2018) 16:24. 10.1186/s12967-018-1392-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 115. Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, et al. Efficacy and Safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, random-ized, double-blind, placebo-controlled clinical trial. Nutrients. (2019) 11:305. 10.3390/nu11020305 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 116. Kobayashi Y, Kinoshita T, Matsumoto A, Yoshino K, Saito I, Xiao JZ. Bifidobacterium breve A1 supplementation improved cognitive decline in older adults with mild cognitive impairment: An open-label, single-arm study. J Prev Alzheimers Dis. (2019) 6:70–5. 10.14283/jpad.2018.32 [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Benef Microbes. (2019) 10:511–20. 10.3920/BM2018.0170 [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Nanri A, Mizoue T, Noda M, Takahashi Y, Kirii K, Inoue M, et al. Magnesium intake and type II diabetes in Japanese men and women: the Japan public health center-based prospective study. Eur J Clin Nutr. (2010) 64:1244–7. 10.1038/ejcn.2010.138 [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Ibsen DB, Levitan EB, Åkesson A, Gigante B, Wolk A. The dietary approach to stop hypertension (DASH) diet is associated with a lower risk of heart failure: A cohort study. Eur J Prev Cardiol. (2022) 29:1114–23. 10.1093/eurjpc/zwac003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Chatterton ML, Mihalopoulos C, O’Neil A, Itsiopoulos C, Opie R, Castle D, et al. Economic evaluation of a dietary inter-vention for adults with major depression (the “SMILES” trial). BMC Public Health. (2018) 18:599. 10.1186/s12889-018-5504-8 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 121. Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: A randomised controlled trial. Br J Nutr. (2008) 99:421–31. 10.1017/S0007114507801097 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Diet and depression: exploring the biological mechanisms of action
Affiliations.
- 1 Deakin University, IMPACT (the Institute for Mental and Physical Health and Clinical Translation), Food & Mood Centre, Geelong, VIC, Australia. [email protected].
- 2 Deakin University, IMPACT (the Institute for Mental and Physical Health and Clinical Translation), Food & Mood Centre, Geelong, VIC, Australia.
- 3 Orygen, The National Centre of Excellence in Youth Mental Health, Centre for Youth Mental Health, Florey Institute for Neuroscience and Mental Health, Melbourne, VIC, Australia.
- 4 Department of Psychiatry, The University of Melbourne, Melbourne, VIC, Australia.
- 5 Deakin University, IMPACT (the Institute for Mental and Physical Health and Clinical Translation), Metabolic Research Unit, Geelong, VIC, Australia.
- 6 Department of Psychological Medicine, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, UK.
- 7 Division of Psychology and Mental Health, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
- 8 NICM Health Research Institute, Western Sydney University, Westmead, NSW, Australia.
- 9 APC Microbiome Ireland, University College Cork, Cork, Ireland.
- 10 Department of Anatomy and Neuroscience, University College Cork, Cork, Ireland.
- 11 Department of Psychiatry and Neurobehavioural Science, University College Cork, Cork, Ireland.
- 12 INFANT Research Centre, University College Cork, Cork, Ireland.
- 13 Deakin University, IMPACT (the Institute for Mental and Physical Health and Clinical Translation), Geelong, VIC, Australia.
- 14 Departments of Psychiatry and Mind-Body Interface Laboratory (MBI-Lab), China Medical University Hospital, Taichung, Taiwan.
- 15 An-Nan Hospital, China Medical University, Tainan, Taiwan.
- 16 College of Medicine, China Medical University, Taichung, Taiwan.
- 17 Department of Psychiatry, Depression Clinical and Research Program, Massachusetts General Hospital, Boston, MA, USA.
- 18 Harvard Medical School, Boston, MA, USA.
- 19 Departments of Neurosurgery and Integrative Biology and Physiology, University of California Los Angeles, Los Angeles, CA, USA.
- 20 Department of Psychiatry & Behavioural Neurosciences, McMaster University, Hamilton, ON, Canada.
- 21 UCLouvain, Université catholique de Louvain, WELBIO-Walloon Excellence in Life Sciences and BIOtechnology, Louvain Drug Research Institute, Metabolism and Nutrition Research Group, Brussels, Belgium.
- 22 Basic and Clinical Neuroscience Department, Institute of Psychiatry Psychology and Neuroscience, King's College London, London, UK.
- 23 Nutrition Research Group, Research Institute of Biomedical and Health Sciences, University of Las Palmas de Gran Canaria, Gran Canaria, Spain.
- 24 Biomedical Research Center Network on Obesity and Nutrition (CIBERobn) Physiopathology of Obesity and Nutrition, Institute of Health Carlos III, Madrid, Spain.
- 25 Université Paris-Saclay, UVSQ, Inserm, CESP, "DevPsy", 94807, Villejuif, France.
- 26 Department of Epidemiology and Public Health, University College London, London, UK.
- 27 Centre for Adolescent Health, Murdoch Children's Research Institute, Melbourne, VIC, Australia.
- 28 Black Dog Institute, Randwick, NSW, Australia.
- 29 James Cook University, Townsville, QLD, Australia.
- PMID: 33144709
- DOI: 10.1038/s41380-020-00925-x
The field of nutritional psychiatry has generated observational and efficacy data supporting a role for healthy dietary patterns in depression onset and symptom management. To guide future clinical trials and targeted dietary therapies, this review provides an overview of what is currently known regarding underlying mechanisms of action by which diet may influence mental and brain health. The mechanisms of action associating diet with health outcomes are complex, multifaceted, interacting, and not restricted to any one biological pathway. Numerous pathways were identified through which diet could plausibly affect mental health. These include modulation of pathways involved in inflammation, oxidative stress, epigenetics, mitochondrial dysfunction, the gut microbiota, tryptophan-kynurenine metabolism, the HPA axis, neurogenesis and BDNF, epigenetics, and obesity. However, the nascent nature of the nutritional psychiatry field to date means that the existing literature identified in this review is largely comprised of preclinical animal studies. To fully identify and elucidate complex mechanisms of action, intervention studies that assess markers related to these pathways within clinically diagnosed human populations are needed.
Publication types
- Depression / genetics
- Depression / metabolism*
- Depression / physiopathology*
- Diet / psychology*
- Epigenesis, Genetic
- Gastrointestinal Microbiome
- Inflammation
- Oxidative Stress
Grants and funding
- MR/N030087/1/MRC_/Medical Research Council/United Kingdom
- MR/S00484X/1/MRC_/Medical Research Council/United Kingdom
- MR/J002739/1/MRC_/Medical Research Council/United Kingdom
- MR/L014815/1/MRC_/Medical Research Council/United Kingdom
- MR/N029488/1/MRC_/Medical Research Council/United Kingdom
- G108/603/MRC_/Medical Research Council/United Kingdom

IMAGES