An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease
Larissa di villeneuve, ive lima souza, fernanda davila sampaio tolentino, renata ferrarotto, gustavo schvartsman.
- Author information
- Article notes
- Copyright and License information
Edited by: Floriana Morgillo, Second University of Naples, Italy
Reviewed by: Susumu Okano, National Cancer Center Hospital East, Japan; Sandro J. Stoeckli, Kantonsspital St. Gallen, Switzerland
*Correspondence: Gustavo Schvartsman [email protected]
This article was submitted to Head and Neck Cancer, a section of the journal Frontiers in Oncology
†These authors share senior authorship
Received 2020 Jul 4; Accepted 2020 Sep 9; Collection date 2020.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Salivary gland carcinomas (SGC) account for less than 5% of head and neck malignant neoplasms, further subcategorized in over 20 histological subtypes. For the most part, treatment for advanced disease is guided by morphology. SGC in general respond poorly to standard chemotherapy, with short durability and significant toxicity. More recently, next-generation sequencing provided significant input on the molecular characterization of each SGC subtype, not only improving diagnostic differentiation between morphologically similar tumor types, but also identifying novel driver pathways that determine tumor biology and may be amenable to targeted therapy. Amongst the most common histological subtype is adenoid cystic carcinoma, which often harbors a chromosome translocation resulting in a MYB-NFIB oncogene, with various degrees of Myb expression. In a smaller subset, NOTCH1 mutations occur, conferring a more aggressive disease and potential sensitivity to Notch inhibitors. Salivary duct carcinomas may overexpress Her-2 and androgen receptor, with promising clinical outcomes after exposure to targeted therapies approved for other indications. Secretory carcinoma, previously known as mammary analogue secretory carcinoma, is distinguished by an ETV6-NTRK3 fusion that can both help differentiate it from its morphologically similar acinar cell carcinoma and also make it susceptible to Trk inhibitors. In the present article, we discuss the molecular abnormalities, their impact on tumor biology, and therapeutic opportunities for the most common SGC subtypes and review published and ongoing clinical trials and future perspectives for this rare diseases.
Keywords: salivary gland cancer, molecular targeted therapy, androgen receptor, immunotherapy, ERBB-2 receptor, gene fusion, drug resistance
Introduction
Salivary gland carcinoma (SGC) is a rare tumor and represents ~6% of head and neck cancers ( 1 ). Malignant tumors of the salivary glands constitute a heterogeneous group of neoplasms that vary depending on the histology and their anatomical location. According to the 2017 WHO Classification, there are 24 malignant histological subtypes ( 2 ). The most prevalent are mucoepidermoid carcinoma (MEC), representing around a third of SGC cases, followed by adenoid cystic carcinoma (ACC), with 23.8% ( 3 ). The parotid gland is the most frequent site of salivary gland tumors, although only 25% of such lesions are malignant. SGC can also originate in the submandibular glands (40–45% of the tumors are malignant), sublingual glands (70–90% are malignant), and minor salivary glands (50% are malignant) ( 4 ).
Treatment for metastatic disease is still mostly based on chemotherapy, despite low response, and survival rates ( 5 ). Currently, encouraging progress in immunohistochemical and molecular alterations, such as the presence of an NTRK fusion, overexpression of Her-2 and androgen receptor, has been made to improve outcomes with targeted therapy.
The aim of this article is to review the main molecular and immunohistochemical characteristics of the most common histological subtypes of SGC, in addition to reviewing current data on biomarker-driven targeted therapy and genomic findings that may be potentially actionable in the future.
Mucoepidermoid Carcinoma
MEC is the most common SGC ( 6 ). In addition to clinical staging, the grade of the tumor is also a prognostic factor in MEC and may guide treatment decision ( 7 ). Despite its prevalence, it is one of the subtypes with the least breakthroughs achieved so far.
A unique t ( 8 , 9 ) translocation, leading to the CRTC1/MAML2 fusion, is present in 56–82% of all MECs ( 10 , 11 ). This fusion protein induces aberrant activation of the Notch signaling pathway, inducing cell proliferation and, therefore, tumor progression ( 12 ). Data on how this abnormality impacts tumor biology and prognosis are conflicting. While some series indicate that fusion-positive MECs were diagnosed at an earlier stage, with lower grade, and a better prognosis ( 8 , 12 , 13 ), other studies do not suggest a prognostic role for the translocation ( 10 , 14 ). CRTC1-MAML2 -positive cells were sensitive to epidermal growth factor receptor ( EGFR ) tyrosine kinase inhibition pre-clinically, suggesting a potential role for such drugs ( 15 ).
The most common genomic abnormalities described in a study of 48 MEC patients were as follows: CDKN2A (41.6%), TP53 (39.6%), CDKN2B (29.2%), BAP1 (20.8%), PIK3CA (20.8%), HRAS (10.4%), BRCA (10.5%) mutations, and ERBB2 amplifications (8.3%) ( 16 ). The latter, though infrequent, may be amenable to Her-2 targeted therapy ( 17 ). TP53 mutation is one of the most common genomic alterations in MEC and is associated with the transformation of low-grade into high-grade tumors ( 12 ). In high-grade MEC, EGFR is overexpressed in 72.7% and was associated with a more aggressive behavior ( 18 ).
Salivary Duct Carcinoma
Salivary duct carcinoma (SDC) is an aggressive subtype of SGC that microscopically resembles high-grade ductal carcinoma of the breast. They can develop as de novo disease or arise from a pleomorphic adenoma (carcinoma ex-pleomorphic adenoma) ( 19 ). The first line of treatment is currently based on platinum chemotherapy, with low response rates and of short duration ( 9 ). Biomarkers of interest have been found within this subtype, showing promising results with targeted therapy.
Androgen receptor (AR) and Her-2 receptors are frequently overexpressed in SDC. In a study of 177 patients with SDC, AR was expressed in 96% of cases ( 20 ). Her-2 overexpression can be found in one third to two thirds of cases, by immunohistochemistry and/or fluorescent in situ hybridization (FISH) ( 20 , 21 ). These markers were not associated with disease biology and prognosis.
As in breast cancer, patients with SDC, and Her-2 overexpression derive benefit from anti-Her-2 therapy. In a phase II study, 57 patients with advanced SDC received docetaxel and trastuzumab, with an objective response rate (ORR) of 70.2%. The median progression-free survival (PFS) was 8.9 months and overall survival (OS) was 39.7 months ( 22 ).
The use of double Her-2 blockade with trastuzumab and pertuzumab was also evaluated in a basket study, which included five patients with advanced, refractory SDC, all with Her-2 amplification/overexpression. Trastuzumab and pertuzumab, without chemotherapy, yielded a partial response in four out of five patients with Her-2-positive SDC (ORR of 80%) ( 23 ).
Ado-trastuzumab emtansine (T-DM1) was also studied in another basket trial, where 10 patients with a median of two previous systemic treatments and HER-2 amplification by next-generation sequencing (NGS) had an ORR of 90%, half of which were complete metabolic responses. Median duration of response and PFS had not been reached with a median follow-up of 12 months ( 24 ). In this same study, the amplification of HER-2 by NGS correlated well with HER2/CEP17 ≥2 by FISH or IHC 3+ ( 24 ).
Treatment with androgen deprivation therapy (ADT) has been proposed after progression to platinum-based chemotherapy when AR is present. In a phase II study, 36 patients with metastatic or locally advanced unresectable SGC, being 34 SDCs, received combined androgen blockade with the luteinizing hormone-releasing hormone (LHRH) analog leuprorelin associated with bicalutamide, with an ORR of 41.7%. The median PFS was 8.8 months and median OS was 30.5 months. The treatment was well-tolerated, with a low rate of toxicity ( 25 ). ADT was also studied in the adjuvant setting in a retrospective study. Stage IVA/B, AR-positive SDC patients who underwent a complete tumor resection received bicalutamide, an LHRH analog or a combination of both. The treatment was associated with a statistically significant increase in the 3-year disease-free survival when compared to a control group (48.2 vs. 27.7%) ( 26 ). A randomized phase II study comparing the efficacy and safety of ADT with platinum-based chemotherapy as first-line therapy for patients with metastatic SDC and AR expression is ongoing ( NCT01969578 ).
Enzalutamide, a more selective AR inhibitor, was given as monotherapy to patients with AR-positive SGC in a phase II trial ( 27 ). The majority (85%) of patients had SDC and 32.6% had prior AR-directed therapy. This study showed that 7 out of 46 patients (15%) had a partial response as best response, but only 4% (2/46) maintained the response until 8 weeks, thus failing to meet its primary endpoint. Therefore, we favor the administration of an antiandrogen agent in combination with an LHRH analog.
The experience of patients with prostate cancer can again be used in patients with SDC. Mechanisms of AR blockade resistance have been discovered in castration-resistant prostate cancer patients. The AR isoform splice variant 7 (AR-V7) results in a truncated receptor that lacks the binding site for androgen, activated even in the absence of ligands and stimulating tumor growth. Detection of AR-V7 in circulating tumor cells from patients with castrate-resistant metastatic prostate cancer was associated with worse PFS and OS in patients who received abiraterone or enzalutamide ( 28 ). In salivary duct carcinomas, the prevalence of AR-V7 is high, varying between 48 and 70% ( 29 , 30 ). Interestingly, it is frequently detected in treatment-naive patients, as opposed to a mechanism of resistance to ADT as in prostate cancer. Therefore, its role in ADT sensitivity in SDC patients remains to be established, warranting further biomarker analysis in future trials. One case report of a patient with AR-positive SDC who expressed AR-V7 did not show response to second-line hormonal therapy with abiraterone ( 31 ).
Other potentially targetable pathways found in 28 SDC patients include TP53 (68%), HRAS (25%), PIK3CA (18%), NF1 (18%), PTEN (10%), BRAF (7%), and NOTCH1 (7%). In the same study, patients did not have common predictive biomarkers of response to immunotherapy: 82% were PD-L1 negative, 91% had a low tumor mutational burden, and no patients presented microsatellite instability ( 29 ). Tipifarnib, a potent inhibitor of farnesyltransferase, an enzyme required for downstream signaling in HRAS-mutated tumors, was evaluated in 12 patients with SGC, with 4 being SDC, none of whom achieved a response. A single patient with acinic cell carcinoma had a partial response lasting at least 14 months ( 32 ).
Secretory Carcinoma
Secretory carcinoma (SC), formerly known as mammary analog secretory carcinoma (MASC), was first described by Skálóva et al. a decade ago ( 33 ). It shows morphological, genetic, and immunohistochemical similarities with breast secretory carcinoma ( 34 ). One of the main differential diagnoses is acinic cell carcinoma (AcCC), which typically contains a basophilic cytoplasm with periodic acid-Schiff-positive zymogen granules and a more diverse cytologic profile compared to SC ( 35 ). SC has several architectural patterns (microcystic, solid, tubular, and cribriform), an abundant and eosinophilic cytoplasm, uniform proliferation and positivity for vimetin, mammaglobin, and S-100 protein in immunohistochemistry ( 36 ). The presence of a chromosomal translocation, t (12, 15) , between the ETV6 gene on chromosome 12 with NTRK3 on chromosome 15, generates the fusion product ETV6 – NTRK3 . It can be detected with a high specificity by reverse-transcriptase polymerase chain reaction (RT-PCR), NGS, or FISH, being the gold standard methods for the diagnosis of this subtype ( 33 , 34 ). Nuclear pattern of pan-Trk immunohistochemistry staining has a good sensitivity to detect an ETV6–NTRK3 fusion, thus aiding in differentiating SC from AcCC. However, it may miss other less frequent ETV6-X fusions, only detected by FISH or RT-PCR ( 37 ).
SC is more commonly found in men (55%), with a mean age of 44 years and mostly arising in the parotid gland, followed by several head and neck locations, including the oral cavity, submandibular glands, soft palate, buccal mucosa, base of tongue, and lips ( 38 ). It usually presents with an indolent clinical course and a good prognosis ( 39 ). Though a few cases of SC with high-grade histology and aggressive behavior have been described in association with ETV6-MET and ETV6-RET fusions, it has not yet been possible to correlate these recently described fusions with an overall behavioral pattern and disease prognosis ( 40 , 41 ).
An NTRK fusion provides an actionable target for this disease by the Trk inhibitors larotrectinib and entrectinib. The benefit of larotrectinib was demonstrated by a phase II study including 12 cases of SC, with an objective response in 10 cases and an ORR of 80% by investigator's assessment ( 42 ). Entrectinib's activity was demonstrated by an integrated analysis of three phase I and II clinical trials (ALKA-372-001, STARTRK-1, and STARTRK-2), with the presence of seven (13%) cases of SC, which demonstrated an objective response in six of the seven cases (86%) ( 43 ). Both drugs received a tissue-agnostic FDA approval for tumors harboring an NTRK fusion.
Mechanisms of acquired resistance to larotrectinib have been described with an on-target mutation in the drug-binding site ( 42 , 44 ). Selitrectinib (LOXO-195), a second-generation Trk inhibitor, was designed to overcome the acquired resistance to the first-line treatment. A phase I/II trial is ongoing ( NCT03215511 ) and has evaluated 29 patients so far, with an ORR of 34% ( 45 ).
Adenocarcinoma, Not Otherwise Specified
Adenocarcinoma, not otherwise specified (NOS), presents as a particularly difficult diagnosis to establish. It is characterized by the presence of areas of glandular or ductal differentiation mixed with a variety of specific growth patterns ( 46 ). Therefore, it is an exclusion diagnosis. The literature is controversial regarding its incidence among SGCs, ranging from 5 to 25% ( 3 , 47 ). They are highly malignant tumors, with an overall 15-year survival rate of 3%, associated with early development of distant metastases and limited treatment options ( 48 ). Since this entity can share some characteristics of other SGCs, it is important to test for actionable biomarkers, such as AR and HER-2. Despite at a lower prevalence, they may be present and predict responses to targeted therapy ( 26 , 49 ).
Immunotherapy in Non-Adenoid Cystic Carcinoma
SGCs seem particularly resistant to immune checkpoint inhibitors. However, they represent a rather heterogeneous group of diseases that may behave differently in regard to the immune system. Linxweiler et al. demonstrated a distinct behavioral pattern in the different subtypes of SGCs. SDC exhibited higher levels of immune infiltration, T-cell dysfunction, and higher mutational load, whereas ACC presented with an overall lower mutational burden and an immune-excluded environment ( 50 ). PD-L1 expression was found to be associated with inferior disease-free survival ( 51 ).
Clinically, the KEYNOTE-028 study, a phase Ib basket trial, treated 26 patients with PD-L1-positive SGC with pembrolizumab at 10 mg/kg every 2 weeks. The low rate of PD-L1 positivity (<30%) limited patient accrual in the screening phase. Patients had adenocarcinoma, NOS (38%), mucoepidermoid (12%), undifferentiated (8%), squamous cell (8%), and ACC (8%). Despite being a PD-L1-enriched cohort, the results were overall disappointing, with an ORR of 12%. There were only three partial responses (two in adenocarcinoma, NOS and one in a high-grade serous carcinoma). The median PFS was 4 months (95% CI: 2 to 5 months) and median OS was 13 months (95% CI: 6 months to not reached) ( 52 ).
Another programmed-death 1 (PD-1) inhibitor is being evaluated in an ongoing phase II trial (NISCAHN trial). The use of nivolumab in 52 non-ACC patients demonstrated a 6-month non-progression rate (NPR 6M ) in 7 patients (14%, 90% CI: 6.8–24.7), with 2 partial responses (3.8%) and 22 patients with stable disease (42.3%). The median PFS was only 1.8 months (95% CI: 1.7–3.5) ( 53 ).
The role of tumor mutation burden (TMB) is unclear in SGCs. The subgroup analysis by TMB from the KEYNOTE-158 trial led to the approval of pembrolizumab for patients with TMB >10 mut/Mb as an agnostic treatment. There were three patients with salivary histologies and high TMB, one of whom achieved a partial response ( 54 ).
The addition of vorinostat, a histone deacetylase (HDAC) inhibitor, to pembrolizumab was evaluated in a phase I/II trial with 25 SGC patients. The association yielded a partial response in 4 patients (16%) and stable disease in 14 (56%), with a median PFS of 6.9 months and a median OS of 14 months ( 55 ). The combination of nivolumab and ipilimumab is being evaluated in an ongoing phase II study ( NCT02834013 ). A summary of all relevant trials in non-ACC histologies is displayed in Table 1 , and ongoing studies are shown in Table 2 . We acknowledge the challenge in treating advanced SGC and propose practical alternatives to chemotherapy based on biomarkers in daily practice, displayed in Figure 1 .
All available data about advanced non-ACC therapy.
ORR, overall response rate; mPFS, median progression free survival; N/A, not available; NR, not reached; NOS, adenocarcinoma; not otherwise specified .
Clinical ongoing trials in different types of non-ACC.
GnRH, gonadotropin-releasing hormone; AR, androgen receptor .
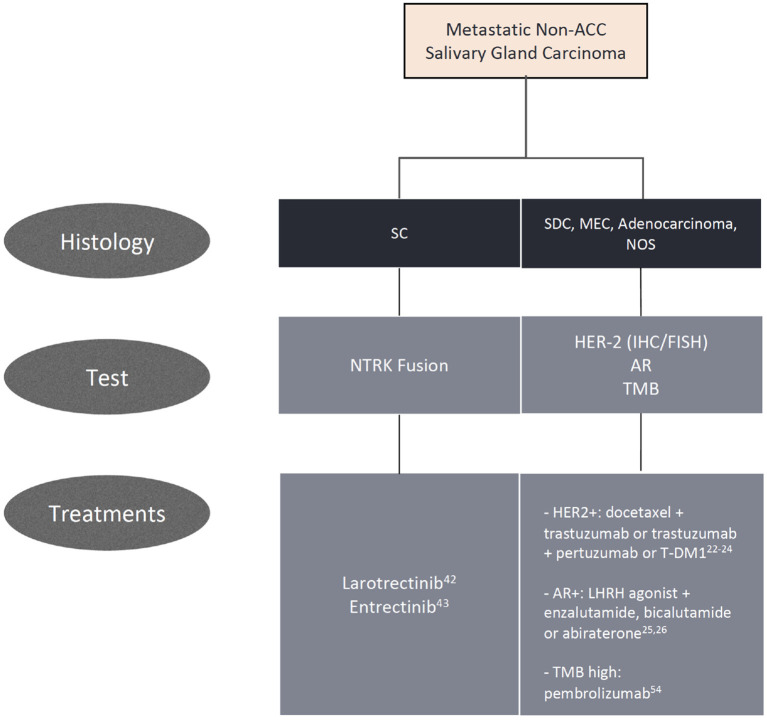
Algorithm for biomarker testing and treatment options in non-adenoid cystic carcinomas.
Adenoid Cystic Carcinoma
ACC is the second most common malignant salivary neoplasm, accounting for around one quarter of cases. It is more frequently diagnosed in females, affecting all age groups and often arising from the minor salivary glands ( 3 , 67 ).
ACC usually has an indolent course, albeit difficult to eradicate due to its persistent nature and recurrent growth pattern, with predilection for perineural invasion. The literature demonstrates that 5-year disease-free survival in patients with ACC is only 30–40% ( 67 ). ACC commonly metastasizes to lungs, bones, and liver, with a median OS of 20–32 months in this setting ( 68 ).
While surgery, with or without postoperative radiotherapy, is the mainstay treatment for localized disease, systemic therapy is reserved to the metastatic or unresectable locally advanced setting, with poor response rates and no consensus about the proper timing to be initiated. In this section, we will review proliferation pathways, molecular insights, and the development of new targeted drugs for patients with advanced disease. Though several actionable pathways are under scrutiny, limited evidence can aid in clinical practice. We propose a practical approach for newly diagnosed advanced ACC and options for later lines of therapy in Figure 2 . Ongoing clinical trials are displayed in Table 3 and a summary of the main ACC studies conducted to date are displayed in Table 4 .
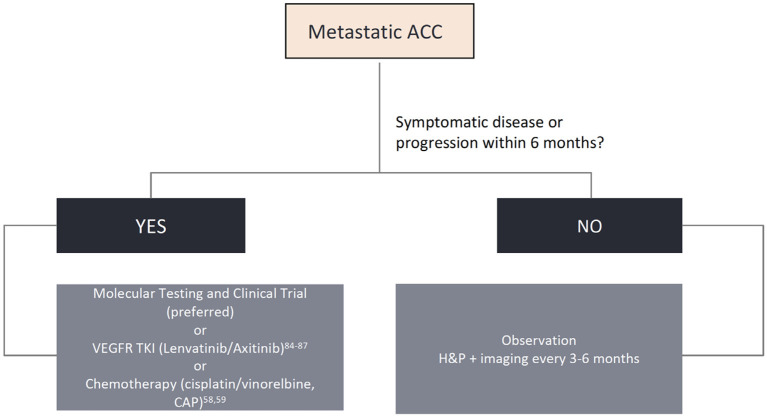
Algorithm for biomarker testing and treatment options in adenoid cystic carcinomas.
Clinical ongoing trials in ACC.
Available data about advanced ACC therapy.
Trials ongoing with preliminary results .
Chemotherapy
Despite response rates of <30%, chemotherapy remains one of the most used treatments for this condition ( 85 ). The most consolidated regimen consists of cisplatin, doxorubicin and cyclophosphamide (CAP) ( 86 ). The best time to start treatment remains controversial, though it is commonly deferred until either symptomatic disease or a more accelerated growth pattern. Other cytotoxic agents have also been shown to be minimally active, such as mitoxantrone and vinorelbine, though other drugs such as paclitaxel should be avoided as single agents due to lack of proven efficacy ( 85 ).
MYB–NFIB Pathway
Myb, a nuclear transcription factor, is overexpressed in 60–80% of ACCs, usually correlated with a genetic translocation of the MYB gene to the transcription factor gene NFIB , resulting in the MYB-NFIB fusion, an important oncogene (t [6, 9] ). This fusion has been postulated as the main driver of tumor proliferation in ACC ( 87 , 88 ). The Myb protein has an N-terminal DNA-binding domain and a central transactivation domain that regulate genes involved in cell cycle control, such as NSR, MET, EGFR, IGF1R , and specifically IGF2 ( 89 ). The latter, by autocrine stimulation, controls the expression of the MYB-NFIB fusion in ACC cells, increasing proliferation and generating changes in the cell cycle and RNA processing ( 89 – 91 ). Other MYB -related fusions were described, however at lower frequencies than MYB-NIFB . Myb overexpression can also occur in the absence of detectable genetic alterations, implying that unknown pathways may be involved in its expression at the protein level ( 89 ).
Pre-clinical studies evaluated the role of targeted therapies, such as linsitinib (Igf1r inhibitor), gefitinib (EGFR inhibitor), and crizotinib (Alk and Met inhibitor) in vitro both as monotherapies and as a triplet regimen. Individually, none showed encouraging results, whereas a significant reduction of Myb expression was seen with the triplet regimen, suggesting a potential clinical benefit ( 92 ). In vivo studies are necessary to confirm activity in clinical practice with a tolerable toxicity profile, a major concern of combining these drugs.
More recently, the use of transretinoic acid (ATRA) showed interesting results in pre-clinical models. The drug reduced Myb binding in intensifying regions in MYB -translocated patient-derived xenograft models, thereby reducing the positive feedback for Myb overexpression cycle and thus reducing tumor proliferation ( 93 ). Two clinical trials are underway to address its role in treating patients with advanced ACC ( NCT03999684 ; NCT04433169 ). Additionally, a study evaluating a Myb vaccine in combination with a novel anti-PD-1 is being conducted ( NCT03287427 ).
NOTCH1, 2, 3
Notch are transmembrane proteins that bind to neighboring cells and activate a biochemical cascade that gives rise to the process of cell differentiation, in addition to acting in the process of lateral regulation, proliferation, and angiogenesis of cells through the MAPK pathways ( 87 ). Mutations in the NOTCH gene family, particularly NOTCH1 , are present in around 20% of ACC patients and are potential oncogenic drivers. The presence of this mutation characterizes a population with more advanced disease, along with the presence of bone and liver metastases and worse outcomes compared to a wild-type population ( 94 ).
A phase I trial tested the efficacy of brontictuzumab (OMP-52M51), a humanized monoclonal antibody against the Notch1 protein in a basket trial for solid tumors. Twelve patients (25%) had a diagnosis of ACC, with two developing a partial response and three with stable disease as best response, with tolerable adverse events ( 81 ). Another phase Ib/II study is evaluating the role of amcasertib (BBI-553), a cancer stemness kinase inhibitor that impairs cancer stem cell survival, which is intimately related to deregulated Notch pathway activity ( 95 ). Preliminary results demonstrated a disease control rate of 86% and median overall survival of 28.3 months ( 82 ). AL101, a γ-secretase inhibitor, also works by inhibiting the Notch pathway during the cleavage process for Notch's protein action in the intracellular domain. A phase I basket trial revealed a partial response lasting 8 months in 1 of 2 patients with ACC accrued ( 83 ). The phase II trial ACCURACY ( NCT03691207 ) for ACC patients bearing NOTCH activating mutations is ongoing. A trial with another Notch inhibitor, CB103, is also being conducted ( NCT03422679 ).
Immunotherapy in ACC
The ACC cohort of the aforementioned KEYNOTE-028 represented only 8% of patients ( N = 2), with none achieving a response. In terms of PFS and OS, results were poorer than with chemo or targeted therapy ( 52 ). Similarly, the combination of pembrolizumab in association with vorinostat was also disappointing in treatment of salivary gland tumors, including ACC, with low response rates ( 55 ). Nivolumab as a single agent was also evaluated in SGCs. In the ACC cohort, an ORR of 8.7% was observed (4/46 patients) ( 53 ). The combination of ipilimumab and nivolumab was initially thought to improve outcomes; however, only 2 out of 32 patients treated achieved a partial response, with a median PFS of 19.3 weeks in a prospective study ( 96 ). As previously stated, ACC appears to lack immune infiltration and harbors a lower mutation burden, being unlikely to benefit from immunotherapy ( 50 ).
EGFR Pathway
EGFR is commonly overexpressed in ACC, though its presence in normal salivary gland tissue precludes any conclusions in its role in cancer development. Mutations in genes related to the EGFR pathway, including EGFR, RAS family, PIK3CA, BRAF , and AKT1 are also present in ACC ( 97 ). Activating mutations in EGFR can be found in 10% of cases, though unlikely to be driver oncogenes in this setting ( 98 ). A phase I study tested gefitinib at 250 mg/day in 18 patients with ACC, and no responses were observed ( 60 ). Cetuximab was also evaluated in a single-arm, phase II study of EGFR-overexpressing patients, with disappointing results ( 59 ). Lapatinib has also been studied in patients who showed overexpression of EGFR and/or Her-2, again with unremarkable outcomes. Clinical benefit with stable disease was achieved in 36% of patients, with no objective responses ( 58 ).
PRMT5 is an enzyme that methylates arginines in proteins important for tumor growth and development ( 99 ). The phase I basket trial METEOR-1 evaluated the role of GSK3326595, a potent and selective PRMT5 inhibitor. Of the selected patients, 14 (26%) had metastatic ACC. Clinical activity was observed in several tumor types, notably with partial responses observed in 3/14 ACC cases, with tolerable adverse events ( 79 ).
Histone Deacetylation
Epigenetic changes were found in most studies that carried out NGS. The acetylation of histone pathways, with mutations in chromatin remodeling genes, such as SMARCA2, CREBBP , and KDM6A , suggests aberrant epigenetic regulation in ACC oncogenesis ( 100 ). A pre-clinical study combining cisplatin and vorinostat found a remarkable efficacy in depleting CSCs and reducing tumor viability in all ACC primary cells ( 101 ). A phase II trial of vorinostat in ACC showed a partial response in 2/30 patients and stable disease in another 27 patients ( 80 ). However, a phase II trial combining vorinostat and pembrolizumab for recurrent or metastatic salivary gland cancer, as aforementioned, showed disappointing results, likely reflecting the immune-tolerant environment of ACC ( 55 ).
Other overexpressed potential target receptors in ACC are the vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor 1 (FGFR1). These are well-established oncogenic pathways and can be inhibited by anti-VEGFR/FGFR drugs ( 102 ). Sorafenib, nintedanib, axitinib, regorafenib, dovitinib, and other multi-kinase inhibitors were tested and showed only a modest benefit, with few objective response rates ( Table 4 ). Notably, lenvatinib was evaluated in a population with metastatic ACC, who had already received up to one line of chemotherapy. A total of 28 patients were enrolled in the study, and 11.5% showed a partial response ( 72 ). Additionally, 25 to 27% of patients with ACC had at least 20% reduction in target lesion size. The median PFS and OS were 9.1 and 27 months, respectively. Despite the encouraging results, 50% of the patients presented grade 3 toxicity and dose reductions were necessary in most of the study population. Similarly, Tchekmedyian et al. conducted another phase II study with lenvatinib, with a 15.6% ORR and a remarkable median PFS of 17.2 months ( 73 ). Axitinib is another multi-kinase inhibitor with interesting results in ACC, but with a lower ORR and median PFS (9.1% and 5.7 months, respectively) ( 70 ). More recently, the first randomized phase II trial of its kind showed a significant improvement in PFS with axitinib vs. observation (HR: 0.25; 95% CI: 0.14–0.42; P < 0.0001), but with no improvement in OS (HR: 0.6; 95% CI: 0.26–1.38; P = 0.23) ( 103 ). In this study, none of the 27 patients treated achieved a response, but all (100%) had stable disease. This rekindles the discussion of whether deferring treatment until a more symptomatic or aggressive course of disease remains acceptable. We favor the use of lenvatinib due to its numerical superiority in ORR and PFS compared to axitinib, but starting at a lower dose of 20 mg/day, with subsequent dose escalation if adequately tolerated.
Despite the high percentages (90%) of overexpression of c-Kit by IHC in ACC, targeted agents such as imatinib and dasatinib failed to show a meaningful activity in this disease ( 65 , 75 , 76 , 104 ). The best response was stable disease in 50% of the patients treated with dasatinib ( 65 ). The disappointing outcomes likely result from the lack of an underlying gene amplification and/or a KIT activating mutation, such as seen in other malignancies (gastrointestinal stromal tumors and chronic myeloid leukemia).
177 Lu-PSMA
ACC cells can express prostate-specific membrane antigen (PSMA) in over 90% of cases, with significant uptake in PSMA-PET/CT ( 105 ). Such as in prostate cancer, this can be useful not only for staging and surveillance but also as an opportunity for PSMA-directed therapy. Lutetium-177 ( 177 Lu)-PSMA is a radiolabeled small molecule that binds with high affinity to PSMA, enabling beta particle therapy targeted to metastatic castration-resistant prostate cancer, with promising results in this tumor type ( 106 ). A single case report so far has been reported in ACC, with a transient pain relief after one dose. However, the patient died within 6 weeks due to a highly refractory and advanced tumor ( 107 ). An ongoing clinical trial is prospectively evaluating the role of 177 Lu-PSMA in advanced ACC ( NCT04291300 ).
Conclusions
In conclusion, SGCs may be challenging to treat due to its several histological subtypes. Molecular diagnostics are able to aid in diagnosis and guide discovery for subtype-specific targeted therapy. Currently, significant efforts are being undertaken to improve outcomes for advanced disease with biomarker-driven research. Given the limited efficacy with chemotherapy, a more personalized approach is of utmost importance to move forward in the management of this infrequent entity.
Author Contributions
LD, IS, FT, RF, and GS participated in the concept design, writing, review, and approval of the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- 1. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. (2005) 114:806–16. 10.1002/ijc.20740 [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al. World Health Organization Classification of Tumours of Head and Neck. Lyon: IARC; (2017). [ Google Scholar ]
- 3. Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. (2008) 44:407–17. 10.1016/j.oraloncology.2007.05.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L, et al. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. (2010) 74:134–48. 10.1016/j.critrevonc.2009.10.004 [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Alfieri S, Granata R, Bergamini C, Resteghini C, Bossi P, Licitra LF, et al. Systemic therapy in metastatic salivary gland carcinomas: a pathology-driven paradigm? Oral Oncol. (2017) 66:58–63. 10.1016/j.oraloncology.2016.12.016 [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Coca-Pelaz A, Rodrigo JP, Triantafyllou A, Hunt JL, Rinaldo A, Strojan P, et al. Salivary mucoepidermoid carcinoma revisited. Eur Arch Otorhinolaryngol. (2015) 272:799–819. 10.1007/s00405-014-3053-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck. (2014) 36:158–63. 10.1002/hed.23256 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Morita M, Murase T, Okumura Y, Ueda K, Sakamoto Y, Masaki A, et al. Clinicopathological significance of EGFR pathway gene mutations and CRTC1/3-MAML2 fusions in salivary gland mucoepidermoid carcinoma. Histopathology. (2020) 76:1013–22. 10.1111/his.14100 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Nakano K, Sato Y, Sasaki T, Shimbashi W, Fukushima H, Yonekawa H, et al. Combination chemotherapy of carboplatin and paclitaxel for advanced/metastatic salivary gland carcinoma patients: differences in responses by different pathological diagnoses. Acta Otolaryngol. (2016) 136:948–51. 10.3109/00016489.2016.1170876 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Saade RE, Bell D, Garcia J, Roberts D, Weber R. Role of CRTC1/MAML2 translocation in the prognosis and clinical outcomes of mucoepidermoid carcinoma. JAMA Otolaryngol Head Neck Surg. (2016) 142:234–40. 10.1001/jamaoto.2015.3270 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Luk PP, Wykes J, Selinger CI, Ekmejian R, Tay J, Gao T, et al. Diagnostic and prognostic utility of mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 121:530–41. 10.1016/j.oooo.2016.01.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Nachtsheim L, Arolt C, Dreyer T, Meyer MF, Brobeil A, Gamerdinger U, et al. Mucoepidermoidcarcinoma – importance in molecular pathology. Laryngo Rhino Otol. (2020) 99:144–8. 10.1055/a-1083-6805 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Anzick SL, Chen WD, Park Y, Meltzer P, Bell D, El-Naggar AK, et al. Unfavorable prognosis of CRTC1- MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosomes Cancer. (2010) 49:59–69. 10.1002/gcc.20719 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Birkeland AC, Foltin SK, Michmerhuizen NL, Hoesli RC, Rosko AJ, Byrd S, et al. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. (2017) 68:5–8. 10.1016/j.oraloncology.2017.02.025 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Chen Z, Chen J, Gu Y, Hu C, Li JL, Lin S, et al. Aberrantly activated AREG–EGFR signaling is required for the growth and survival of CRTC1–MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene. (2014) 33:3869–77. 10.1038/onc.2013.348 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Wang K, McDermott JD, Schrock AB, Elvin JA, Gay L, Karam SD, et al. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequent BAP1, PIK3CA, and other actionable genomic alterations. Ann Oncol. (2017) 28:748–53. 10.1093/annonc/mdw689 [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. De Block K, Vander Poorten V, Dormaar T, Nuyts S, Hauben E, Floris G, et al. Metastatic HER-2-positive salivary gland carcinoma treated with trastuzumab and a taxane: a series of six patients. Acta Clin Belg. (2016) 71:383–8. 10.1080/17843286.2016.1173940 [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Lujan B, Hakim S, Moyano S, Nadal A, Caballero M, Diaz A, et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer. (2010) 103:510–6. 10.1038/sj.bjc.6605788 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Simpson RH. Salivary duct carcinoma: new developments–morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol. (2013) 7 (Suppl. 1):S48–58. 10.1007/s12105-013-0456-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Boon E, Bel M, van Boxtel W, van der Graaf WTA, van RJJ, Eerenstein Es SEJ, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from the Netherlands. Int J Cancer. (2018) 143:758–66. 10.1002/ijc.31353 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. McHugh JB, Visscher DW, Barnes EL. Update on selected salivary gland neoplasms. Arch Pathol Lab Med. (2009) 133:1763–74. 10.1043/1543-2165-133.11.1763 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. (2019) 37:125–34. 10.1200/JCO.18.00545 [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. (2018) 36:536–42. 10.1200/JCO.2017.75.3780 [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Li BT, Shen R, Offin M, Buonocore DJ, Myers ML, Venkatesh A, et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): results from a phase II basket trial. J Clin Oncol. (2019) 37:6001. 10.1200/JCO.2019.37.15_suppl.6001 [ DOI ] [ Google Scholar ]
- 25. Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. (2018) 29:979–84. 10.1093/annonc/mdx771 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Van Boxtel W, Locati LD, van Engen-van Grunsven ACH, Bergamini C, Jonker MA, Fiets E, et al. Adjuvant androgen deprivation therapy for poor-risk, androgen receptor-positive salivary duct carcinoma. Eur J Cancer. (2019) 110:62–70. 10.1016/j.ejca.2018.12.035 [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Ho AL, Foster NR, Zoroufy AJ, Worden FP, Price KA, Adkins D, et al. Alliance A091404: a phase II study of enzalutamide (NSC# 766085) for patients with androgen receptor-positive salivary cancers. J Clin Oncol. (2019) 37 (15 Suppl):6020. 10.1200/JCO.2019.37.15_suppl.6020 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. (2019) 37:1120–9. 10.1200/JCO.18.01731 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Gargano SM, Senarathne W, Feldman R, Florento E, Stafford P, Swensen J, et al. Novel therapeutic targets in salivary duct carcinoma uncovered by comprehensive molecular profiling. Cancer Med. (2019) 8:7322–9. 10.1002/cam4.2602 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Yang RK, Zhao P, Lu C, Luo J, Hu R. Expression pattern of androgen receptor and AR-V7 in androgen deprivation therapy naive salivary duct carcinomas. Hum Pathol. (2018) 84:173–82. 10.1016/j.humpath.2018.09.009 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Cappelletti V, Miodini P, Reduzzi C, Alfieri S, Daidone MG, Licitra L, et al. Tailoring treatment of salivary duct carcinoma by liquid biopsy: ARv7 expression in circulating tumor cells. Ann Oncol. (2018) 29:1598–600. 10.1093/annonc/mdy141 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Ho AL, Hanna GJ, Scholz CR, Gualberto A, Park SH. Preliminary activity of tipifarnib in tumors of the head and neck, salivary gland and urothelial tract with HRAS mutations. J Clin Oncol. (2020) 38:6504. 10.1200/JCO.2020.38.15_suppl.6504 [ DOI ] [ Google Scholar ]
- 33. Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. (2010) 34:599–608. 10.1097/PAS.0b013e3181d9efcc [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Balanzá R, Arrangoiz R, Cordera F, Muñoz M, Luque-de-León E, Moreno M, et al. Mammary analog secretory carcinoma of the parotid gland: a case report and literature review. Int J Surg Case Rep. (2015) 16:187–91. 10.1016/j.ijscr.2015.09.031 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Parekh V, Stevens TM. Mammary analogue secretory carcinoma. Arch Pathol Lab Med. (2016) 140:997–1001. 10.5858/arpa.2015-0075-RS [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Fehr A, Löning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol. (2011) 35:1600–2. 10.1097/PAS.0b013e31822832c7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Hung YP, Jo VY, Hornick JL. Immunohistochemistry with a pan-TRK antibody distinguishes secretory carcinoma of the salivary gland from acinic cell carcinoma. Histopathology. (2019) 75:54–62. 10.1111/his.13845 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Sethi R, Kozin E, Remenschneider A, VanderLaan P, Faquin W, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. (2014) 124:188–95. 10.1002/lary.24254 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Montalvo N, Galarza D, Redrobán L. Secretory carcinoma of the parotid: making the correct diagnosis of a rare salivary gland carcinoma when molecular biology testing is not available. Case Rep Pathol. (2019) 2019:5103496. 10.1155/2019/5103496 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Rooper LM, Karantanos T, Ning Y, Bishop JA, Gordon SW, Kang H. Salivary secretory carcinoma with a novel ETV6-MET fusion expanding the molecular spectrum of a recently described entity. Am J Surg Pathol. (2018) 42:1121–6. 10.1097/PAS.0000000000001065 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Skalova A, Vanecek T, Martinek P, Weinreb I, Stevens TM, Simpson RHW, et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation report of 10 cases. Am J Surg Pathol. (2018) 42:234–46. 10.1097/PAS.0000000000000972 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Drilon A, Laetsch T, Kummar S, DuBois S, Lassen U, Demetri G, et al. Efficacy of larotrectinib in TRK fusion– positive cancers in adults and children. N Engl J Med. (2018) 378:731–9. 10.1056/NEJMoa1714448 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Doebele R, Drilon A, Paz-Ares L, Siena S, Shaw A, Farago A, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet. (2020) 21:271–82. 10.1016/s1470-2045(19)30691-6 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Hemming M, Nathenson M, Lin J, Shaolin M, Du Z, Malik K, et al. Response and mechanisms of resistance to larotrectinib and selitrectinib in metastatic undifferentiated sarcoma harboring oncogenic fusion of NTRK . JCO Precis Oncol. (2020) 4:79–90. 10.1200/PO.19.00287 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Hyman D, Kummar S, Farago A, Geoerger B, Mau-Sorensen M, Taylor M, et al. CT-127 - phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) [abstract]. In: Proceedings of the 10th Annual Meeting of the American Association for Cancer Research; 2019. Philadelphia, PA: AACR; (2019). p. 127. [ Google Scholar ]
- 46. Li J, Wang BY, Nelson M, Li L, Hu Y, Urken ML, et al. Salivary adenocarcinoma, not otherwise specified: a collection of orphans. Arch Pathol Lab Med. (2004) 128:1385–94. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Reinheimer A, Vieira DS, Cordeiro MM, Rivero ER. Retrospective study of 124 cases of salivary gland tumors and literature review. J Clin Exp Dent. (2019) 11:e1025–32. 10.4317/jced.55685 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Psarris A, Koufopoulos N, Grivas A, Papatheodorou DC, Khaldi L. Tumor to tumor metastasis from adenocarcinoma not otherwise specified of the parotid gland to uterine leiomyoma: presentation of a unique case. Cureus. (2020) 12:e6789. 10.7759/cureus.6789 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Wang K, Russell JS, McDermott JD, Elvin JA, Khaira D, Johnson A, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. (2016) 22:6061–8. 10.1158/1078-0432.CCR-15-2568 [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Linxweiler M, Kuo F, Katabi N, Lee M, Nadeem Z, Dalin MG, et al. The immune microenvironment and neoantigen landscape of aggressive salivary gland carcinomas differ by subtype. Clin Cancer Res. (2020) 26:2859–70. 10.1158/1078-0432.CCR-19-3758 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Mukaigawa T, Hayashi R, Hashimoto K, Ugumori T, Hato N, Fujii S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol. (2016) 114:36–43. 10.1002/jso.24266 [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Cohen RB, Delord JP, Doi T, Piha-Paul SA, Liu SV, Gilbert J, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol. (2018) 41:1083–8. 10.1097/COC.0000000000000429 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Fayette J, Even C, Digue L, Geoffrois L, Rolland F, Cupissol D, et al. NISCAHN: a phase II, multicenter nonrandomized trial aiming at evaluating nivolumab (N) in two cohorts of patients (pts) with recurrent/metastatic (R/M) salivary gland carcinoma of the head and neck (SGCHN), on behalf of the unicancer head and neck group. J Clin Oncol. (2019) 37:6083. 10.1200/JCO.2019.37.15_suppl.6083 [ DOI ] [ Google Scholar ]
- 54. Marabelle A, Fakih MG, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. 1192OAssociation of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Ann Oncol. (2019) 30:v477–8. 10.1093/annonc/mdz253.018 [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Rodriguez CP, Wu QV, Voutsinas JM, Fromm JP, Jiang X, Pillarisetty VG, et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res. (2020) 26:837–45. 10.1158/1078-0432.CCR-19-2214 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. (2003) 39:724–7. 10.1016/S1368-8375(03)00097-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Jhaveri KL, Wang XV, Makker V, Luoh SW, Mitchell EP, Zwiebel JA, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. (2019) 30:1821–30. 10.1093/annonc/mdz291 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. (2007) 25:3978–84. 10.1200/JCO.2007.11.8612 [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol. (2009) 45:574–8. 10.1016/j.oraloncology.2008.07.010 [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Jakob JA, Kies MS, Glisson BS, Kupferman ME, Liu DD, Lee JJ, et al. Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck. (2015) 37:644–9. 10.1002/hed.23647 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Locati LD, Cavalieri S, Bergamini CI, Resteghini CI, Alfieri S, Calareso G, et al. Phase II trial with axitinib in recurrent and/or metastatic salivary gland cancers of the upper aerodigestive tract. Head Neck. (2019) 41:3670–76. 10.1002/hed.25891 [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Locati LD, Perrone F, Cortelazzi B, Bergamini C, Bossi P, Civelli E, et al. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: translational analyses and clinical impact. Eur J Cancer. (2016) 69:158–65. 10.1016/j.ejca.2016.09.022 [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Guigay J, Fayette J, Even C, Cupissol D, Rolland F, Peyrade F, et al. PACSA: phase II study of pazopanib in patients with progressive recurrent or metastatic (R/M) salivary gland carcinoma (SGC). J Clin Oncol. (2016) 4:6086. 10.1200/JCO.2016.34.15_suppl.6086 [ DOI ] [ Google Scholar ]
- 64. Kim Y, Lee SJ, Lee JY, Lee SH, Sun JM, Park K, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: a multicenter phase 2 study (Korean cancer study group HN14-01). Cancer. (2017) 123:1958–64. 10.1002/cncr.30537 [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Wong SJ, Karrison T, Hayes DN, Kies MS, Cullen KJ, Tanvetyanon T, et al. Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors. Ann Oncol. (2016) 27:318–23. 10.1093/annonc/mdv537 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. (2013) 18:294–300. 10.1634/theoncologist.2012-0369 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Nascimento AG, Amaral Amaral ALP. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. (1986) 57:312–19. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. (2002) 24:779–83. 10.1002/hed.10126 [ DOI ] [ PubMed ] [ Google Scholar ]
- 69. Thomson DJ, Silva P, Denton K, Bonington S, Mak SK, Swindell R, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. (2015) 37:182–7. 10.1002/hed.23577 [ DOI ] [ PubMed ] [ Google Scholar ]
- 70. Ho AL, Dunn L, Sherman EJ, Fury MG, Baxi SS, Chandramohan R, et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann Oncol. (2016) 27:1902–8. 10.1093/annonc/mdw287 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Ho AL, Sherman EJ, Baxi SS, Haque S, Ni A, Antonescu CR, et al. Phase II study of regorafenib in progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. (2016) 34:6–20. 10.1200/JCO.2016.34.15_suppl.6096 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Locati LD, Galbiati D, Calareso G, Alfieri S, Singer S, Cavalieri S, et al. Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: activity and quality of life. Cancer. (2020) 126:1888–94. 10.1002/cncr.32754 [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Tchekmedyian V, Sherman EJ, Dunn L, Tran C, Baxi S, Katabi N, et al. A phase II study of lenvatinib in patients with progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. (2018) 36:6022. 10.1200/JCO.2018.36.15_suppl.6022 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Dillon PM, Petroni GR, Horton BJ, Moskaluk CA, Fracasso PM, Douvas MG, et al. A phase II study of dovitinib in patients with recurrent or metastatic adenoid cystic carcinoma. Clin Cancer Res. (2017) 23:4138–45. 10.1158/1078-0432.CCR-16-2942 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a princess margaret hospital phase II consortium study. J Clin Oncol. (2005) 23:585–90. 10.1200/JCO.2005.06.125 [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Guigay MJ, Bidault F, Temam S, Janot F, Raymond E, Faivre S. Antitumor activity of imatinib in progressive, highly expressing KIT adenoid cystic carcinoma of the salivary glands: a phase II study. J Clin Oncol. (2007) 25:6086. 10.1200/jco.2007.25.18_suppl.608616135502 [ DOI ] [ Google Scholar ]
- 77. Kim DW, Oh DY, Shin SH, Kang JH, Cho BC, Chung JS, et al. A multicenter phase II study of everolimus in patients with progressive unresectable adenoid cystic carcinoma. BMC Cancer. (2014) 14:795. 10.1186/1471-2407-14-795 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Argiris A, Ghebremichael M, Burtness B, Axelrod RS, Deconti RC, Forastiere AA. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: a trial of the eastern cooperative oncology group (E1303). Cancer. (2011) 117:3374–82. 10.1002/cncr.25852 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 79. Siu LL, Rasco DW, Vinay SP, Romano PM, Menis J, Heinhuis KM, et al. Meteor-1: a phase I study of GSK3326595, a first-in-class proteins arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumors. Ann Oncol. (2019) 30:59–193. 10.1093/annonc/mdz244 [ DOI ] [ Google Scholar ]
- 80. Gonçalves PH, Heilbrun LK, Barrett MT, Kummar S, Hansen AR, Siu LL, et al. A phase 2 study of vorinostat in locally advanced, recurrent, or metastatic adenoid cystic carcinoma. Oncotarget. (2017) 8:32918–29. 10.18632/oncotarget.16464 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Ferrarotto R, Eckhardt G, Patnaik A, LoRusso P, Faoro L, Heymach JV, et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. (2018) 29:1561–8. 10.1093/annonc/mdy171 [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Cote GM, Edenfield WJ, Laurie SA, Chau NG, Becerra C, Spira AI, et al. A phase 1b/2 study of amcasertib, a first-in-class cancer stemness kinase inhibitor, in advanced adenoid cystic carcinoma. J Clin Oncol. (2017) 35:6036. 10.1200/JCO.2017.35.15_suppl.6036 [ DOI ] [ Google Scholar ]
- 83. El-Khoueiry AB, Desai J, Iyer SP, Gadgeel SM, Ramalingam SS, Horn L, et al. A phase I study of AL101, a pan-NOTCH inhibitor, in patients (pts) with locally advanced or metastatic solid tumors. J Clin Oncol. (2018) 36:2515. 10.1200/JCO.2018.36.15_suppl.2515 [ DOI ] [ Google Scholar ]
- 84. Even C, Lassen UN, Merchan JR, Torneau CL, Soria JC, Ferte C, et al. Notch pathway inhibition with LY3039478 in adenoid cystic carcinoma (ACC). J Clin Oncol. (2017) 35:6024. 10.1200/JCO.2017.35.15_suppl.6024 [ DOI ] [ Google Scholar ]
- 85. Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. (2011) 12:815–24. 10.1016/S1470-2045(10)70245-X [ DOI ] [ PubMed ] [ Google Scholar ]
- 86. Licitra L, Cavina R, Grandi C, Palma SD, Guzzo M, Demicheli R, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol. (1996) 7:640–2. 10.1093/oxfordjournals.annonc.a010684 [ DOI ] [ PubMed ] [ Google Scholar ]
- 87. Allen S, Ho Ochoa A, Jayakumaran G, Zehir A, Mayor CV, Tepe J, et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest. (2019) 129:4276–89. 10.1172/JCI128227 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Xu LH, Zhao F, Yang WW, Chen CW, Du ZH, Fu M, et al. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int J Oncol. (2019) 54:1579–90. 10.3892/ijo.2019.4754 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Andersson MK, Åman P, Stenman G. IGF2/IGF1R signaling as a therapeutic target in MYB-positive adenoid cystic carcinomas and other fusion gene-driven tumors. Cells. (2019) 8:913. 10.3390/cells8080913 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. Wang Y, Zhang CY, Xia RH, Han J, Sun B, Sun SY, et al. The MYB/miR-130a/NDRG2 axis modulates tumor proliferation and metastatic potential in salivary adenoid cystic carcinoma. Cell Death Dis. (2018) 9:917. 10.1038/s41419-018-0966-2 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 91. Almeida-Pinto YD, Costa SFDS, de Andrade BAB, Altemani A, Vargas PA, Abreu LG, et al. t(6;9)(MYB-NFIB) in head and neck adenoid cystic carcinoma: a systematic review with meta-analysis. Oral Dis. (2019) 25:1277–82. 10.1111/odi.12984 [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Andersson MK, Afshari MK, Andrén Y, Wick MJ, Stenman G. Targeting the oncogenic transcriptional regulator MYB in adenoid cystic carcinoma by inhibition of IGF1R/AKT signaling. J Natl Cancer Inst. (2017) 109:djx017. 10.1093/jnci/djx017 [ DOI ] [ PubMed ] [ Google Scholar ]
- 93. Mandelbaum J, Shestopalov IA, Henderson RE, Chau NG, Knoechel B, Wick MG, et al. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J Exp Med. (2018) 10:2673–85. 10.1084/jem.20180939 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to notch1 inhibitors. J Clin Oncol. (2017) 35:352–60. 10.1200/JCO.2016.67.5264 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. Saygin C, Matei D, Majeti R, Reizes O, Lathia JD. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. (2019) 24:25–40. 10.1016/j.stem.2018.11.017 [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Tchekmedyian V, Sherman EJ, Dunn L, Fetten JV, Michel LS, Kriplani A, et al. A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic adenoid cystic carcinoma (R/M ACC). J Clin Oncol. (2019) 37:6084. 10.1200/JCO.2019.37.15_suppl.6084 [ DOI ] [ Google Scholar ]
- 97. Saida K, Murase T, Ito M, Fujii K, Takino H, Masaki A, et al. Mutation analysis of the EGFR pathway genes, EGFR, RAS, PIK3CA, BRAF , and AKT1 , in salivary gland adenoid cystic carcinoma. Oncotarget. (2018) 9:17043–55. 10.18632/oncotarget.24818 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. (2010) 16:2266–74. 10.1158/1078-0432.CCR-09-0238 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. Tada Y, Kokabu S, Sugiyama G, Nakatomi C, Aoki K, Fukushima H, et al. The novel IκB kinase β inhibitor IMD-0560 prevents bone invasion by oral squamous cell carcinoma. Oncotarget. (2014) 5:12317–30. 10.18632/oncotarget.2640 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 100. Stephens PJ, Davies HR, Mitani Y, Loo PV, Shlien A, Tarpey PS, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. (2013) 123:2965–8. 10.1172/JCI67201 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 101. Almeida LO, Guimarães DM, Martins MD, Martins MAT, Warner KA, Nör JE, et al. Unlocking the chromatin of adenoid cystic carcinomas using HDAC inhibitors sensitize cancer stem cells to cisplatin and induces tumor senescence. Stem Cell Res. (2017) 21:94–105. 10.1016/j.scr.2017.04.003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 102. Lim JJ, Kang S, Lee MR, Pai HK, Yoon HJ, Lee JI, et al. Expression of vascular endothelial growth factor in salivary gland carcinomas and its relation to p53, Ki-67 and prognosis. J Oral Pathol Med. (2003) 32:552–61. 10.1034/j.1600-0714.2003.00073.x-i1 [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Keam B, Kang EJ, Ahn MJ, Ock CY, Lee KW, Kwon JH, et al. Randomized phase II study of axitinib vs. observation in patients with recurred or metastatic adenoid cystic carcinoma. J Clin Oncol. (2020) 38:6503. 10.1200/JCO.2020.38.15_suppl.6503 [ DOI ] [ PubMed ] [ Google Scholar ]
- 104. Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. (2007) 43:33–6. 10.1016/j.oraloncology.2005.12.026 [ DOI ] [ PubMed ] [ Google Scholar ]
- 105. van Boxtel W, Lütje S, van Engen-van Grunsven ICH, Verhaegh GW, Schalken JA, Jonker MA, et al. 68 Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: a phase 2 imaging study. Theranostics. (2020) 10:2273–83. 10.7150/thno.38501 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. (2018) 19:825–33. 10.1016/S1470-2045(18)30198-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Simsek DH, Kuyumcu S, Agaoglu FY, Unal SN. Radionuclide therapy with 177Lu-PSMA in a case of metastatic adenoid cystic carcinoma of the parotid. Clin Nucl Med. (2019) 44:764–6. 10.1097/RLU.0000000000002645 [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (637.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Malignant salivary gland tumors.
Allen Young ; Oluwafunmilola T. Okuyemi .
Affiliations
Last Update: January 12, 2023 .
- Continuing Education Activity
Salivary gland tumors are a rare group of complex, heterogeneous histologies located in the parotid gland, submandibular gland, sublingual gland, and minor salivary glands of the upper aerodigestive tract. This diverse group of malignant tumors has a wide range of etiology, pathophysiology, treatment, and prognosis. This activity reviews the evaluation and treatment of malignant salivary gland tumors and highlights the role of the interprofessional team in evaluating and treating patients with this condition.
- Identify the etiology of malignant salivary gland tumors and associated conditions.
- Assess the physical examination of patients with malignant salivary gland tumors.
- Evaluate the treatment options available for malignant salivary gland tumors.
- Introduction
Salivary gland tumors are a rare group of complex, heterogeneous histologies located in the parotid, submandibular, sublingual, and minor salivary glands of the upper aerodigestive tract. The wide variety of tumor etiology, microscopic histology, growth patterns, and tumor characteristics can make diagnosis and treatment challenging for clinicians. The World Health Organization in 2005 recognized 24 different malignant salivary gland cancers; the most common histologies include mucoepidermoid carcinoma (MEC), acinic cell carcinoma (ACC), adenoid cystic carcinoma (AdCC), carcinoma ex-pleomorphic adenoma (CExPA), and adenocarcinoma. [1]
The exact etiology of salivary gland cancer is unknown. Still, various mechanisms have been proposed, including radiation, viruses (EBV and HIV), immunosuppression, ultraviolet light exposure, occupational exposures in rubber or nickel industries, prior diagnosis of medulloblastoma, prior diagnosis of basal cell carcinoma, androgen receptor expression, and genetics. [2] [3] [4] [5] [6] [7] [8] In studies involving Japanese survivors of the atomic bomb and patients who received radiation treatment during childhood for benign conditions, radiation exposure was identified as a significant risk factor for the development of salivary malignancies. [9] [10] MEC appears to be the most common salivary gland malignancy associated with radiation exposure. [11] [12] Compared to other head and neck cancers, the risk of developing malignant salivary gland tumors from exposure to tobacco and alcohol has been controversial, with several studies noting both positive association and no appreciable association. Sawabe et al found no significant association between cigarette smoking and MEC but did show a positive correlation between tobacco smoking and other malignant salivary histologies. [13] [14] Chronic inflammation of the salivary glands has not been established as a risk factor. However, autoimmune conditions such as Sjogren syndrome may predispose an individual to develop a salivary gland malignancy such as lymphoma. [15] [16] [17] [18] Although squamous cell carcinoma (SCC) of the head and neck has been linked to tobacco, alcohol, and UV exposure, the presence of primary SCC in the salivary glands is rare and does not retain the same risk factors. [19] The most significant risk factor for primary SCC of the salivary glands appears to be prior gland radiation. [20] [21]
Most melanoma cases of the major salivary glands are attributed to metastasis from cutaneous sources of the upper face and scalp. [22] However, the presence of melanoma in the parotid without any other primary sites has been reported in the literature. [23] Although the exact etiology is unknown, melanocytes have been found in the intralobular duct of the parotid gland and have been postulated to serve as the cells of origin for primary melanoma of the salivary glands. [24] [25] The development of non-Hodgkin lymphoma (NHL) of the salivary glands has been associated with a prior diagnosis of the autoimmune disease, Sjogren syndrome (SS). Studies have shown that 4.3% of patients with Sjogren syndrome develop NHL within 5-10 years. [26] [27]
- Epidemiology
Salivary gland malignancies comprise 0.5 to 1.2% of all cancers and 5% of head and neck cancers. [28] [29] [30] They more commonly affect women with a male-to-female ratio of 1 to 1.5. [31] Malignant lesions are found in about 21.7% of all salivary gland neoplasms. [32] [33] Most malignant cases occur in the parotid, followed by the submandibular, sublingual, and minor salivary glands. [34] The probability of malignancy in a parotid mass ranges from 15% to 32%, compared to 41% to 50% in a submandibular mass, 70% to 90% in minor salivary gland masses, and almost 100% in sublingual masses. [35] Salivary gland tumors in children are more likely to be malignant. Malignant tumors in children under 10 years old tend to be of a higher grade with poorer prognosis. [36] Salivary tumors in children older than 10 were benign in 85% of cases, similar to that of the adult population. [37] The most common pediatric malignant salivary gland tumors include MEC, adenocarcinoma, and ACC. [28] [30]
The parotid gland harbors 60 to 75% of all salivary gland tumors. [38] The most common malignant tumors are MEC, AdCC, CExPA, adenocarcinoma, and SCC. [39] [40] The submandibular gland harbors 10-15% of all salivary gland tumors with an equal distribution of benign and malignant neoplasms. [41] AdCC is the most common malignant neoplasm in the submandibular gland, followed by MEC and CExPA. Less common tumors include ACC, salivary duct carcinoma, epi-myoepithelial carcinoma, carcinosarcoma, oncocytic carcinoma, and SCC. [41] Malignant submandibular tumors are more common in the 6th decade with a predilection for men. [42] In the minor salivary glands, as many as 50% of tumors are malignant, most often located in the palate. [38]
Epidemiology of Specific Salivary Gland Malignancies
- MEC is the most common salivary gland malignancy in adults and children. [36] About 89% of cases are found in the parotid, followed by 8.4% in the submandibular gland and 0.4% in the sublingual gland. [43] There is an equal distribution between the sexes with a predilection for the 4th to 5th decade. [44]
- AdCC accounts for about 10% of all salivary gland neoplasms and 30% of all minor salivary gland tumors. It has a predilection for patients in the 5th to 6th decade with no difference in gender, although it tends to be more common in the submandibular gland in women. [36] [45] [46]
- ACC is located in the parotid gland in more than 80% of all cases, submandibular glands in 4%, and intraoral minor salivary glands in 17%. Bilateral parotid involvement is seen in 3-5% of cases. [47] It has a predilection for women and more commonly occurs in the 5th decade. [36] [44] [46]
- CExPA accounts for 5% to 15% of all salivary gland malignancies and can arise in up to 25% of untreated pleomorphic adenomas. [48] Malignant transformation is often seen in recurrent pleomorphic adenoma (PA), with the risk of transformation ranging from 5% to 10% for untreated pleomorphic adenomas over 15 years. [41] About 82% of cases occur in the parotid and submandibular glands, followed by 18% in the intraoral minor salivary glands. [41]
- Polymorphous Low-Grade Adenocarcinoma (PLGA) occurs almost exclusively in the minor salivary glands, with rare reports of it in the major salivary glands. [49] It is the second most common intraoral minor salivary gland malignancy after MEC. [49] [50]
- Salivary Duct Carcinoma accounts for 7 to 10% of all salivary gland tumors and is often found in the parotid in older men in the 6th to 7th decades. [30] [36] [51] It is a very aggressive malignancy of the salivary glands.
- SCC has a predilection for men and is usually found in the parotid. They are considered rare due to the infrequent occurrence of squamous metaplasia of ductal epithelium, which is thought to be responsible for the malignant transformation. [52] The specific etiology of the malignant transformation is not known, although there is evidence implicating high-risk HPV viruses. [53] High-grade MEC and extension from an extra-parotid source are often misdiagnosed as primary SCC of the parotid. The true incidence of primary squamous cell carcinoma of the parotid is unknown due to its rarity and its frequency of being a misclassification of metastatic SCC. Evidence from the literature suggests the true incidence may be around 0.75-1%. [54] Although a range of 0.3% to 4.3% has also been cited, these higher frequencies are thought to be due to misrepresentations or misclassification of these tumors. [52] [55] [52]
- Primary melanoma of the salivary gland is extremely rare and accounts for 0.68% of malignant parotid neoplasms. [56] There is a predilection for males in the 6th to 7th decade. [19] [57] [58] Most melanoma in the parotid is due to cutaneous and mucosal metastasis from the head and neck. [59] [60]
- NHL of the salivary gland accounts for less than 10% of malignant salivary gland tumors. [61] [62] [63] Although it encompasses less than 5% of all extranodal NHL, it is the most common extranodal lymphoma at the neck, comprising two-thirds of all cases. [64] It preferentially occurs in women and patients over 50 years old. [65] The most common variant of lymphoma associated with Sjogren Syndrome is mucosa-associated lymphoid tissue lymphoma (MALT), with 48% to 75% of all cases followed by diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma. [27] [61] [66] The majority of cases occur in the parotid gland. [26] [67] [68]
- Pathophysiology
MEC arises from the epithelium of the interlobular and intralobular salivary ducts. The most common genetic finding is the chromosomal translocation t(11;19)(q21:p13), leading to the fusion of MECT1 and MAML2 genes, which is responsible for disrupting the NOTCH signaling pathway. [69] [70] This translocation is found in 50% to 70% of patients with MEC and is more often seen in low-grade tumors associated with a better prognosis. [71] [72] Over 50% of AdCC tumors contain the t(6;9)(q22-23;p23-24) translocation, which fuses the MYB protooncogene on chromosome 6q to the NFIB gene on chromosome 9p, resulting in an overexpression of MYB-NFIB fusion oncogene and worse prognosis. [73] [74] [75] ACC develops from tumorigenesis of cells responsible for acinar development, namely the reserve cells of the intercalated ducts and terminal tubules. In mice studies, inactivation of PTEN and Apc led to an upregulation of mTOR and Wnt signaling, which increased the incidence of ACC. [76] ACC in humans has also been found to have a higher mTOR expression than other salivary malignancies. [8]
The presence of biological receptors in salivary gland malignancies has recently come under investigation. Tyrosine kinase receptors such as the epidermal growth factor receptor (EGFR) are present in up to 71% of all salivary gland cancers. [77] Epidermal growth factor receptor 2 (HER2) is present in cancers derived from excretory ducts such as the salivary ducts. [78] C-kit is expressed in malignancies derived from the intercalated ducts of salivary glands such as AdCC. [79] Androgen receptor expression has been found in salivary duct carcinoma and adenocarcinoma. [77] Gonadal hormone receptors such as the estrogen and progesterone receptors have been found in benign and malignant salivary gland neoplasms. [80] Salivary duct carcinoma tends to overexpress erb-B2 , which has been associated with a worse prognosis. [81] [82] Epigenetic mutations involving DNA promoter methylation of tumor suppressor genes can lead to transcriptional inactivation and increase both the risk of salivary duct carcinoma and the transformation of pleomorphic adenoma into carcinoma ex pleomorphic adenoma. [83] [84] The presence of SCC in the salivary glands is suspected to arise from several sources, including 1) malignant squamous portion of MEC, 2) metastasis from a cutaneous or mucosal head and neck source, 3) metastasis from distant primary carcinoma, or 4) primary squamous cell carcinoma. [19]
Approximately 4.4% to 5.2% of malignant melanoma of the parotid cases do not have a primary source. [57] [58] They are suspected to be due to a metastatic cutaneous source that has subsequently regressed or metastases from an unusual mucosal site such as the sclera, nasal cavity, paranasal sinuses, or throat. [85] [22] [86] [85] In addition, although rare, primary melanoma can develop in the parotid gland. Melanoblasts can migrate into the gland during the invagination of oral epithelium during parotid gland development. [87] In an autopsy case, Takeda et al discovered the presence of melanocytes in the basal and suprabasal layers of the interlobular ducts, which can serve as the cells of origin for primary melanoma of the parotid. [25] The development of lymphoma from Sjogren syndrome is theorized to be due to prolonged B-cell activity and survival. Sjogren syndrome is associated with excessive expression of cytokines, chemokines, and inflammatory factors such as interferon (IFN) and B-cell activating factor. [88] [89] The B-cell activating factor induces the migration of T and B lymphocytes into the salivary glands to create an autoimmune reaction. The B cells start producing SS-A and SS-B antibodies, which are used to diagnose Sjogren syndrome. [27] Although the exact pathway is unknown, the constant activity of these B cells is believed to be the inciting event of lymphomagenesis in Sjogren syndrome. [90]
- Histopathology
MEC is a non-encapsulated, poorly circumscribed mass with cystic components that can often be mistaken for mucoceles. [91] MEC can comprise up to 6 different types of cells, including maternal, intermediate, epidermoid, clear, columnar, and mucous. Maternal cells are the progenitors for the other cell types and have small, round nuclei with scant, basophilic cytoplasm. Intermediate cells can develop into either glandular or epidermoid cells. Epidermoid cells present with homogenous cytoplasm and occasional keratin pearls and intracellular bridges. Clear cells have watery cytoplasm with small, central nuclei. Columnar cells resemble the secretory duct cells of the salivary gland and transform into mucous cells. Mucous cells have small nuclei at the periphery with foamy, reticular cytoplasm and can occasionally develop a signet-ring appearance. [91] [92] MEC can occur as low, intermediate, or high-grade disease depending on the quantity of aggressive epidermoid tissue. Low-grade MEC has well-formed cystic spaces with high glandular composition. The intermediate grade tends to have more solid nests of epidermoid and intermediate cells with less prominent cystic spaces. High-grade MEC has limited mucous cells with large amounts of solid, squamous cells that can often be misdiagnosed as squamous cell carcinoma. [93] [94] When MEC arises in the submandibular gland, it behaves aggressively regardless of its histologic grading. [36]
AdCC presents as a non-encapsulated, well-circumscribed mass with biphasic ductal and myoepithelial components and 3 distinct patterns: tubular, cribriform, and solid. [36] [30] The cribriform variant presents a “Swiss-cheese” appearance, containing cylindrical pseudocysts lined with epithelial cells and hyaline material. The tubular variant consists of ducts lined by 1 to 2 layers of myoepithelial-like cells. The solid variant has solid epithelial islands with central areas of necrosis. [28] [95] The solid variant is the most aggressive of the 3 and tends to hematogenous metastasis in 40-60% of cases with perineural invasion along the palatine branches, through which it can extend into the pterygopalatine fossa and cavernous sinus. [95] [96] [97]
ACC presents several growth patterns, including solid (most common), papillary-cystic, follicular, and microcystic. [98] Microscopically, it appears well-circumscribed by a lymphoid stroma or a capsule. Individual cells can be granulated serous-type cells, primitive tubule cells, or undifferentiated polymorphous cells. [98] [99] Although ACC is usually considered a low-grade malignancy, it can undergo high-grade transformation into high-grade adenocarcinoma or undifferentiated carcinoma and present with complete loss of acinar differentiation, desmoplasia, tumor necrosis, frequent mitosis, and cervical or distant metastasis. [100] [101] [102]
CExPA arises from an existing pleomorphic adenoma and can histologically resemble salivary duct carcinoma, adenocarcinoma, or undifferentiated carcinoma. [48] [103] Microscopically, it can present with varying extents of invasion, including intracapsular, minor extracapsular (<5 mm beyond the capsule), and wide extracapsular (>5 mm beyond the capsule). The wide extracapsular variant presents a high risk of recurrence and distant metastasis. [30] [48] [103]
PLGA presents as an unencapsulated mass predominantly seen on the palate, with a high concentration of minor salivary glands. Histologically, it can resemble AdCC and has an infiltrative growth pattern with a mixture of solid, cribriform, tubular, and cystic patterns. [104] [105] [106]
Salivary Duct Carcinoma is similar in histology to high-grade duct carcinoma of the breast. [107] It can present with cribriform, solid, cystic, or papillary growth patterns. [30]
SCC presents with intracellular keratinization, intercellular bridging, and keratin-pearl formation without any intracellular mucin. [92]
Melanoma of the parotid presents with large, rounded eosinophilic cells with a pleomorphic nucleus and prominent nuclei. [24] [108] To be considered a primary melanoma of the parotid, the melanoma tumor must be intra-parotid with no lymph node tissue present and no prior excisions or evidence of malignant lesions elsewhere in the body. [109]
NHL of the major salivary gland presents with atypical lymphocytes with invasion into the adjacent ductal epithelium, lymphoepithelial lesions, and lymphoid follicles or germinal centers. [66] [110] [111]
A high-grade transformation is a rare event involving a well-differentiated tumor's transformation into an aggressive, poorly differentiated, high-grade morphology that lacks any original histologic characteristics. It can be seen in AdCC, ACC, PLGA, and MEC. [112]
- History and Physical
Patients often present with a history of a palpable mass that localizes to the region of the salivary glands. A detailed history should be obtained, including pain symptoms, onset and duration of the mass, growth rate, associated swallowing difficulties, and occurrence of facial weakness. Past medical history, including a history of previous skin cancers of the head and neck, surgical history, family history of malignancies, and social risk factors such as smoking, past radiation exposure, and occupational exposures, should be elicited. Physical exam should include a comprehensive head and neck exam focused on the salivary glands, cranial nerves, and cervical nodes. Malignant tumors can present as painless, fixed, or mobile masses, so distinguishing from benign tumors may be hard to distinguish. Advanced malignancies may present with pain, palatal fullness, parapharyngeal fullness, trismus, overlying skin ulceration, or fistulas. Minor salivary gland tumors can present as submucosal oral swelling with ulceration or with nasal obstruction and bleeding if they occur in the nasal cavity or nasopharynx. Minor salivary gland tumors in the pharynx or larynx can cause dysphagia, odynophagia, airway obstruction, vocal hoarseness, and dyspnea on exertion. [113] Rapid growth, pain, facial nerve paresis, and cervical lymphadenopathy are concerning signs of malignancy. [38]
Parotid malignancies presenting as large, fixed preauricular masses can be associated with cervical lymph node metastasis. Facial nerve paralysis can be seen in up to 12 to 15% of cases and is often associated with AdCC, MEC, and salivary duct carcinoma. [114] Submandibular malignancies often present as painless neck masses in the submandibular triangle of the neck. Malignant lesions here are often firm and lobulated, may be fixed to skin or deeper tissues, and may exhibit associated paralysis of the facial nerve's lingual nerve, hypoglossal nerve, or marginal mandibular branch. [115] Sublingual gland malignancies can present as painless, non-ulcerated masses on the floor of the mouth, although about 50% of cases can present with pain and numbness. [116] As previously mentioned, minor salivary gland malignant tumors tend to present along the upper aerodigestive tract as asymptomatic submucosal masses, rare ulceration, and occasional upper airway narrowing. [36]
Specific presentations peculiar to the salivary malignancies include:
- MEC often presents as an asymptomatic, firm mass with occasional pain and facial paralysis. [36] First-bite syndrome has been associated with MEC. [114]
- AdCC presents as a slow-growing, solid mass with pain secondary to perineural and extra-parenchymal invasion. Cervical lymph node metastasis occurs at a rate of 20% at presentation and is more common with submandibular tumors. [36] First-bite syndrome has also been associated with AdCC. [117]
- ACC presents as a slow-growing, solitary mass with occasional pain. [36]
- PLGA presents as a well-circumscribed mass with pain; ulceration is present in 8% of cases. [36]
- CExPA commonly presents in a patient with a history of a long-standing parotid mass characterized by a sudden increase in size. It can be associated with pain and nerve palsy in up to 30% of cases. [118]
- Salivary duct carcinoma presents as a firm, poorly defined mass with the invasion of the surrounding glandular and soft tissue, resulting in associated pain and facial nerve palsy. More than 50% of patients display cervical metastasis at initial presentation. [36]
- SCC can present as a long-standing mass with sudden rapid growth, pain, facial nerve palsy, or overlying skin ulceration. [119]
- Melanoma of the parotid gland can present as an indolent and firm preauricular mass with infiltrative growth resulting in pain, facial nerve palsy, and overlying dermal changes such as ulceration and erythema. [19] [120]
- NHL of the salivary gland can present with unilateral or bilateral swelling of the parotid gland, cervical lymphadenopathy, splenomegaly, vasculitis, and palpable purpura. [27]
Evaluation Options Include
Ultrasound is the first non-invasive option for evaluating major salivary gland tumors, especially superficial parotid lesions. It can help localize tumors, distinguish solid masses from cystic collections, and help guide fine-needle aspiration biopsy. Heterogenous echogenicity, local invasion, poorly defined margins, and lymphadenopathy are sonographic signs of malignancy. [121] [122]
Computerized tomography (CT). Conventional CT can evaluate tumor extent, bony infiltration, and lymphadenopathy. However, it is limited by the dental artifact and has a poor soft-tissue resolution, especially for MEC, AdCC, and ACC, leading to underestimating the lesion. [30]
Magnetic resonance imaging (MRI) . MRI is recommended to assess tumor extent, soft tissue invasion, and nerve involvement for lesions in the deep parotid lobe, sublingual glands, and minor salivary glands. MRI can be performed to detect the facial nerve branches and their interface with surrounding soft tissue for surgical planning. MRI has a higher sensitivity and specificity than CT in detecting perineural spread, especially for AdCC. [123] Heterogenous contrast enhancement, local soft tissue invasion, poorly defined margins, hypointensity on T2-weighted imaging, and lymphadenopathy are characteristics of malignancy. [121] [124] Diffusion-weighted MRI can quantify the diffusion properties of water in tumor tissue into the apparent diffusion coefficient (ADC) that can help distinguish malignant tumors from benign tumors. Salivary gland malignancies have significantly smaller ADC than benign tumors, although the ADC of Warthin’s tumor is even smaller than that of malignancies due to excessive lymphoid tissue resembling lymphoma. [125] [126]
Positron emission tomography (PET). The role of PET is to detect locoregional and distant metastasis. Compared with conventional CT, PET is more accurate in demonstrating tumor extension, nodal involvement, local recurrence, and distant metastasis due to the tissues’ higher standardized uptake values. [127] [128] However, PET cannot differentiate between benign and malignant tumors because benign tumors (such as pleomorphic adenoma and Warthin’s tumor) exhibit high glucose uptake values due to cells with high mitochondrial content. [129] [130]
Biopsy. Imaging is unable to completely distinguish between benign and malignant lesions. Therefore, obtaining histological samples is key to determining the next steps in management. An incisional biopsy can be used for minor salivary glands in the oral cavity but is not recommended for parotid lesions due to the risk of damage to the facial nerve and the possibility of tumor seeding. Hence, ultrasound-guided fine-needle aspiration (FNA) is preferred. [131] FNA's sensitivity and specificity in differentiating between benign and malignant lesions are 80% and 97%, respectively. [132] However, FNA may falter in determining the specific malignant subtype and tumor grade. [133]
An ultrasound-guided core needle biopsy can obtain larger tissue specimens with histologic architecture, improving tumor grade recognition to allow further subtyping. The diagnosis and classification of the specific type of lymphoma of the salivary glands require histological cellular architecture. [134] Disadvantages of core biopsy include more pain, increased risk of facial nerve injury, and hematoma. [135] The intraoperative frozen section has a sensitivity and specificity of 90% and 99%, distinguishing between benign and malignant lesions. [136]
- Treatment / Management
Surgical excision with negative margins is the mainstay of treatment for all salivary gland malignancies. Despite preoperative attempts to obtain a diagnosis through a needle biopsy, histological diagnosis may sometimes not be available until surgery, when frozen specimens can be analyzed. The extent of surgery and the need for neck dissection or adjuvant radiotherapy depend on the malignancy's subtype, grade, and stage. [30] Adjuvant radiation therapy is recommended after surgical excision to improve locoregional control in cases with positive margins, cervical metastases, advanced cancer stage, aggressive histology or grade, perineural invasion, lymphovascular invasion, or extra-glandular extension. [137] [138] [139] [140] [141] Primary radiation therapy is usually reserved for patients with unresectable disease, metastatic disease, or poor surgical candidacy. [142] [143] Chemotherapy and biologically targeted therapy are the standard of care for lymphoma but have limited roles in the other malignancies, often utilized in the palliative setting with partial response. [27] [34] More prospective research and clinical trials are needed before chemotherapy and targeted therapies become routine clinical tools in managing salivary malignancies. [34] [113]
- Differential Diagnosis
The differential diagnosis for malignant salivary gland tumors are as follows:
- Benign salivary lesions (pleomorphic adenoma, myoepithelioma, basal cell adenoma, Warthin's tumor, oncocytoma, canalicular adenoma, sebaceous adenoma, lymphadenoma, inverted ductal papilloma, intraductal papilloma, cystadenoma)
- Benign salivary cysts
- Sialadenitis
- Lymphadenopathy (from infectious or inflammatory causes)
- Tuberculosis
- Mononucleosis
- Chronic Sclerosing Sialadenitis (Küttner tumor, which is more common in the submandibular gland)
- First Branchial cleft cysts
- Lymphoepithelial cysts (especially in immunocompromised patients such as HIV patients)
- Metastases from tumors of other body sites
- Surgical Oncology
Wide local excision is the first-line treatment for all malignant salivary gland tumors. Superficial parotidectomy is the standard treatment of choice for the removal of benign and malignant parotid tumors in the superficial lobe with preservation of the facial nerve. [38] The procedure involves elevating a skin flap over the parotid capsule, identifying the facial nerve trunk and dissecting along all its branches, with preservation of the facial nerve branches that are uninvolved by malignancy, and subsequent removal of the superficial lobe of the parotid while maintaining the integrity of the tumor pseudocapsule. [38] A total parotidectomy is required for advanced or deep lobe lesions. If the facial nerve is infiltrated, a radical total parotidectomy with facial nerve sacrifice and reconstruction is required. [144] For tumors abutting the facial nerve, the tumor can be peeled off and followed with adjuvant radiotherapy to clear microscopic disease. [144] [145]
The disadvantages of superficial parotidectomy include excessive resection of parotid tissue, leading to loss of parotid function, and the risk of facial nerve paralysis due to complete facial nerve dissection. For low-grade or smaller lesions located in the lateral or latero-inferior part of the superficial lobe, the partial superficial parotidectomy, where the tumor is resected with a normal margin of parotid tissue with the facial nerve dissected only in the vicinity of the tumor, has been proposed. [146] [147] A comparison of the 2 techniques was studied by Roh et al who found partial superficial parotidectomy to have better cosmesis, improved sensory and salivary functions, less facial nerve weakness, and no difference in recurrence rate. [148] Excision of the entire submandibular gland should be performed for any submandibular malignant lesions or if the final diagnosis cannot be confirmed. [149] The procedure involves elevating subplatysmal flaps over the submandibular gland, identifying and ligating the facial vessels and submandibular duct, preserving the lingual and hypoglossal nerves, and removing the gland while maintaining the integrity of the tumor.
Cervical (neck) metastases in salivary gland malignancies range from 10% to 40%, depending on tumor grade, location, histologic subtype, and size. [150] [151] Cervical metastasis is present in 35% to 62% of high-grade tumors and 0% to 15% of low-grade tumors. [152] Minor salivary gland tumors in the pharynx or larynx present with neck metastases in 30 to 47% of cases. [153] [154] Histologies such as salivary duct carcinoma, adenocarcinoma, or high-grade MEC have a rate of neck metastasis ranging from 40% to 80%. [150] [153] [155] [156] In patients with a clinically negative neck, occult metastasis has been identified in 12% to 50% of cases. [151] [157] The presence of occult metastasis has been associated with tumor grade, advanced patient age, lymphatic invasion, and extra-parotid tumor extension. [158]
Chisholm et al analyzed the anatomical distributions of positive nodal metastasis in parotid cancers showing only ipsilateral involvement. They found up to 28% in level I, 59% in level II, 52% in level II, 38% in level IV, and 42% in level IV. About 33% of cases displayed skip metastasis to level V, which led to the recommendation of a complete ipsilateral neck dissection from level I-V for parotid malignancies with positive neck disease. [159] [152] [160] In malignancies with clinically negative neck disease, elective neck dissection is recommended for high-grade T3/T4 tumors, facial nerve paralysis, age >54 years, extra-glandular extension, and lymphatic invasion. [71] [161]
For submandibular and minor salivary gland carcinomas, clinical cervical metastasis is present in 8 to 20% of cases. It increases to 41% after neck dissection, with the most frequently involved nodes occurring in levels I-III for submandibular gland malignancies. [42] [139] [151] Thus, a supra-omohyoid neck dissection (level I to III) is recommended. [115] [153] [162] Minor salivary gland carcinomas from the pharynx or larynx should undergo neck dissection from levels II to IV. [154]
- Radiation Oncology
Radiation therapy (RT) to the primary site is recommended in the postoperative or adjuvant setting to improve locoregional control and survival, especially in high-grade histology, advanced stage of the disease, positive or close margins, perineural invasion, lymphovascular invasion, cervical lymph node metastases, or extra-glandular extension. [137] [138] [139] [140] [141] A total dose of 60-66 Gy divided into daily fractions of 2 Gy over 6 weeks has been recommended. Elective neck radiation for clinically negative neck disease is optional. Positive neck disease can be irradiated with 50-60 Gy. [113] Studies show 10-year local control rates ranging from 83% to 91% for combining surgery and adjuvant radiation therapy in advanced-stage disease. [139] [163] [164]
Primary radiation therapy is advised only in selective scenarios, including unresectable disease, poor surgical candidacy, unacceptable functional and cosmetic comorbidities from surgery, and palliation. [139] [143] Mendenhall et al. showed that the 10-year locoregional control rate in the setting of primary radiation therapy was dependent on the disease stage, ranging from 70% for stage I-III down to 24% for stage IV. Less than 20% of patients with stage IV disease are cured with radiation therapy alone. [143] Neutron therapy has better locoregional control rates than photon therapy in unresectable cases, but there appears to be no difference in long-term survival. [165]
- Medical Oncology
Chemotherapy is the mainstay of treatment for lymphoma of the salivary gland. Patients with MALT lymphoma are commonly treated with rituximab, 2-chlorodeoxyadenosine, fludarabine, and chlorambucil-cyclophosphamide. [134] Rituximab is a chimeric monoclonal antibody that binds to the surface CD20 proteins of B cells, triggering cellular death. [27] For cases of DLBCL transformation, the standard chemotherapeutic regimen is cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with rituximab. [166]
The benefit of chemotherapy is limited for other salivary gland malignancies. Combining chemotherapeutic agents (such as carboplatin, paclitaxel, fluorouracil, and hydroxyurea) with adjuvant radiation has shown moderate benefit with 5-year locoregional control rates of 90% to 95% reported in some studies. However, there was no overall survival benefit compared with non-responding patients. [167] [168] [169] Other studies have failed to demonstrate any significant locoregional control or survival benefit in incorporating chemotherapy into primary or adjuvant RT. [34] [170] [171] In patients who are not surgical or radiation candidates, palliative chemotherapy involving cisplatin, paclitaxel, and gemcitabine has been shown to have a partial response in 20 to 25% of non-AdCC cases and <10% of AdCC cases. [172] [173]
Targeted therapies against C-Kit (Imatinib), HER2 (Trastuzumab), EGFR (Cetuximab), and mTOR (Temsirolimus) have gained popularity due to the expression of biological receptors in several malignant salivary tumors. However, response rates have not been superior. This is likely due to a discrepancy between protein overexpression and gene mutation and loss of tumor suppressor PTEN that interferes with target therapy responsiveness. [174] [175] [176] [177] [178] [179]
Clinical Staging
Major salivary gland carcinomas are staged according to the TNM system of the American Joint Committee on Cancer (AJCC) 8 edition. [113] [180] [181]
T Category Tumor Size and Characteristics
Staging for NHL based on the Lugano classification. [182]
Stage Nodal Involvement
Salivary gland malignancies have a wide spectrum of prognoses due to the heterogeneous array of histologies. In general, the prognosis of salivary malignancies is better for children and adolescents than adults due to lower frequencies of cervical metastasis, absence of local soft tissue invasion, and more differentiated histologies. The 5-year overall survival for children after surgical treatment is 85% compared with 60% for adults. [183] [184] Regardless of malignant subtype, negative prognostic factors for survival include advanced age, high-stage disease, high-grade histology, cervical metastasis, male gender, presence of pain, facial nerve involvement, perineural invasion, local soft tissue invasion, positive or close margins, distant metastasis, and comorbidities. [143] [185] [186] [187] [188] [189] [190] [191] Smoking and alcohol statuses were controversial, with some studies showing no significant influence on overall survival or disease-free survival [192] [193] , whereas other studies found them to be poor prognostic factors. [3] [13] Distant metastasis most commonly involves the lungs (40% to 91%) followed by bone (13% to 40%), liver (4% to 19%), soft tissue (9%), distant nodal basins (8%), and the brain (4% to 7%). [194] Distant metastasis is the main cause of mortality for malignant salivary glands. [113]
Overexpression of epidermal growth factor receptor (EGRF) and human epidermal growth factor receptor 2 (HER2) have been identified in several salivary gland malignancies, such as salivary duct carcinoma. They tend to predict a higher incidence of cervical metastasis and worse survival. [30] The absence of c-kit expression in AdCC is correlated with poorer prognosis. [155]
- MEC: Low-grade MEC, especially cases with the MECT1-MAML2 gene fusion, have a good prognosis with a 5-year survival rate of more than 90%. However, the high-grade squamous variant of MEC has a high tendency for recurrence, cervical metastasis, and distant metastasis, with 5-year survival as low as 30% to 41.6%. [187] [195] About 33% of patients with MEC were found to have regional metastasis, of which 85% exhibited a high-grade primary tumor. [196] The 5-year survival has been shown to range from 75% to 96%. [197] [198] [199] The overall 10-year survival rate for MEC is about 53%. [200]
- AdCC: Distant metastasis in AdCC takes a prolonged course and is often more common than cervical metastasis. [41] The rate of distant metastasis ranges from 25% to 55%, whereas the local recurrence rate ranges from 15% to 85%. Distant metastasis from submandibular AdCC most commonly involves the lungs, and regional metastasis from submandibular AdCC is more common than other major salivary glands due to the proximity of the draining lymph nodes. [200] [201] The 5-year survival rate cited in the literature is unreliably optimistic at 60% to 90% due to the prolonged, indolent growth pattern of AdCC that can ultimately result in poor disease-free survival of 10% to 20% at 10 to 15 years. The solid pattern histology has the worst prognosis. [36] [200] [202] [203]
- ACC: Overall survival is influenced by male gender, age >45 years, tumor size larger than 3 cm, and presence of distant metastasis. [204] Cervical neck metastasis occurs in 6-10% and distant metastasis in 12% to 15% of cases. [205] [206] [207] [208] The survival rate at 5-years is 75% to 96% and at 20-years is 56%. [99] [206] [209]
- CExPA: CExPA can display a wide range of recurrence and survival rates depending on the extent of differentiation and invasion. Tumors with wide extracapsular invasion beyond 5 mm of the capsule present a high risk of recurrence and distant metastasis. [30] [48] [103] . Regional metastasis in CExPA reduces the 5-year survival rate from 67% to 16%. [210] Clinical neck metastasis is present in 33% of patients with CExPA, and occult neck metastasis in 16% of patients. [211] The 5-year survival ranges from 30% to 96%, depending on the histology variant. [48] [212] The overall 10-year survival rate for CExPA is 62%. [200]
- PLGA: Local recurrence is reported in 9-33% of cases, with cervical lymph node metastasis present in 6% to 35% of cases and distant metastasis at 1%. [213] PLGA has an indolent growth pattern known to recur for decades after initial treatment. [49] The 5-year survival rate ranges from 75% to 100%. [213] [214] [215]
- Salivary duct carcinoma: Salivary duct carcinoma presents with perineural and lymphovascular invasion in 60% and 30% of cases, respectively. [30] Cervical lymph node metastasis is seen in up to 60% of cases, with 50% of patients already having distant metastasis at the time of presentation. [155] [216] The 5-year overall survival ranges from 20% to 50%. [51] [113] [216] [113] [217] The majority of patients die within 3 years of diagnosis. [51]
- SCC: The overall 5-year survival of primary SCC of the salivary glands is influenced based on tumor staging and ranges from 50% to 80% for early-stage tumors and 15% for advanced-stage tumors. [20] [21] [55] Advanced age, facial palsy, and regional nodal metastasis were poor prognostic factors. [55] [218]
- Melanoma: The prognosis of melanoma of unknown primary is controversial, with some studies stating no difference compared with typical melanoma at the same stage. [58] [86] However, Wang et al showed patients with salivary melanoma of unknown primary had improved 5-year survival at 38.5% compared with 14.2% for patients with known primary tumor sites. [22] Furthermore, melanoma patients with unknown primary sites were found to have a longer disease-free survival period (4.2 years vs. 2.6 years). [22]
- NHL: Patients with MALT have a 3-year overall survival of 80%. [134] Patients with more aggressive DLBCL transformation have a 3-year overall survival ranging from 37% to 100%. [166] Poor prognostic factors include age over 60, elevated serum lactate dehydrogenase level, more than 1 extranodal site, and stage III or IV disease. [219]
- Complications
The incidence of temporary facial nerve palsy after parotidectomy ranges from 10% to 65%, with permanent paralysis seen in less than 5%. [146] [220] [221] The incidence of Frey syndrome after parotidectomy varies widely from 2% to 80% due to the time interval since surgery as well as the surveillance criteria from the surgeons. [222] Treatment for Frey syndrome includes antiperspirant ointment, botulinum toxin A injections, and barrier flaps such as superficial musculoaponeurotic system flap, temporoparietal flap, sternocleidomastoid flap, anterolateral thigh flap, or thick skin flaps. [222] [223] [224] [225] Additional complications from surgical resection include sialocele, salivary fistula, neuromas of the great auricular nerve, and preauricular skin anesthesia. [113] Patients who undergo submandibular gland resection or neck dissection for cervical lymph node metastasis have a risk of neurological damage to the spinal accessory, phrenic, hypoglossal, lingual, vagus, sympathetic trunk, and the marginal mandibular branch of the facial nerve. [226] [227] [228] Complications from radiation include sensorineural hearing loss, chronic otitis media/externa, otalgia, skin erythema, mucositis, dysphagia, dysgeusia, xerostomia, soft tissue fibrosis, osteoradionecrosis, and radiation-induced malignancy. [34] [113] In a study, approximately 36% of patients were found to develop hearing loss of 10 dB or higher at 4kHz. [229] Mandibular osteoradionecrosis (<2%) and radiation-induced malignancy (1%) at 10 to 25 years are rare complications. [113] [230] [231] [232]
- Postoperative and Rehabilitation Care
Postoperative surveillance aims to detect locoregional recurrence and manage any treatment complications that may arise. Surgery and radiation can result in long-term swallowing difficulties (xerostomia, mucositis, trismus), as well as cosmetic defects from facial nerve paralysis. Early intervention for facial nerve reconstruction and rehabilitation and consultation with speech pathologists for swallowing difficulties is vital for allowing the patient to re-integrate as much as possible into their pre-treatment functional status and improve their quality of life. [226] [233] [234] Over 70% of recurrences occur within the first 3 years of treatment, except for low-grade malignancies and AdCC. According to the National Comprehensive Cancer Network (NCCN) guidelines for post-treatment surveillance, patients should receive regular follow up every 1 to 3 months in the 1 year after treatment, every 2-6 months in the 2 years, every 4-8 months in the 3 to 5 years, and every 12 months after the 5 years. [235] All salivary gland malignancies require post-treatment surveillance for up to 20 years (especially AdCC due to its propensity for delayed recurrences or metastases). Annual chest imaging should be performed for high-grade lesions and submandibular gland tumors due to the high risk of pulmonary metastasis. Thyroid hormones should be monitored every 6-12 months for neck radiation patients. [113]
- Consultations
An interprofessional team would need to diagnose and manage salivary malignancies, including an otolaryngologist, general surgeon, plastic or reconstructive surgeon, radiation oncologist, medical oncologist, speech-language pathologist, psychologist, and primary care physician.
- Deterrence and Patient Education
Patients should be educated on the risk factors for developing salivary gland tumors and counseled to avoid exposure to these risks as much as possible. Patients and their families should be educated on the different treatment options and timelines, including surgical resection, radiation therapy, and chemotherapy. Patients and parents should be counseled on the possible risks and complications of all treatment modalities, and their comorbidities should be fully assessed by their primary care physicians to determine if they are candidates for surgical resection. Long-term follow-up is crucial for salivary malignancies and must be emphasized to patients, given the indolent growth pattern of certain pathologies such as AdCC.
- Enhancing Healthcare Team Outcomes
Patients with salivary malignancies should be managed by a multidisciplinary team of otolaryngologists, plastic surgeons, general surgeons, pathologists, radiation oncologists, medical oncologists, speech-language pathologists, psychologists, and primary care physicians. Salivary malignancies present with varying histologies and severities and often require multimodal therapy involving surgery, radiation, and chemotherapy. Close communication and collaboration between the surgeons, radiation oncologists, and medical oncologists can provide a tailored approach for each patient. Difficulties in swallowing can occur from acute radiation toxicity. Patients may need a consultation with general surgery for gastric tube placement to ensure optimal nutrition perioperatively and for the duration of any adjuvant therapy. The speech-language pathologist's early post-treatment intervention can help patients regain pre-treatment function to prevent malnutrition and dependence on enteral feeding. Recurrence of salivary malignancies can occur several years, even decades, after initial treatment. Thus, the otolaryngologist, medical and radiation oncologists, and primary care physician's routine close follow-up is strongly recommended. Finally, patients may develop visible surgical scarring and facial nerve paralysis associated with depression, social anxiety, and social avoidance. Formal peer support groups and consultation with a psychologist can aid patients in addressing these latter concerns.
- Review Questions
- Access free multiple choice questions on this topic.
- Click here for a simplified version.
- Comment on this article.
Disclosure: Allen Young declares no relevant financial relationships with ineligible companies.
Disclosure: Oluwafunmilola Okuyemi declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Young A, Okuyemi OT. Malignant Salivary Gland Tumors. [Updated 2023 Jan 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review A review: Immunological markers for malignant salivary gland tumors. [J Oral Biol Craniofac Res. 2014] Review A review: Immunological markers for malignant salivary gland tumors. Namboodiripad PC. J Oral Biol Craniofac Res. 2014 May-Aug; 4(2):127-34. Epub 2014 Aug 28.
- Common Malignant Salivary Gland Epithelial Tumors. [Surg Pathol Clin. 2011] Common Malignant Salivary Gland Epithelial Tumors. Seethala RR, Barnes EL. Surg Pathol Clin. 2011 Dec; 4(4):1177-215.
- Simultaneous pleomorphic adenoma of the left parotid gland and adenoid cystic carcinoma of the contralateral sublingual salivary gland: a case report. [Oral Maxillofac Surg. 2009] Simultaneous pleomorphic adenoma of the left parotid gland and adenoid cystic carcinoma of the contralateral sublingual salivary gland: a case report. Papadogeorgakis N, Kalfarentzos EF, Vourlakou C, Malta F, Exarhos D. Oral Maxillofac Surg. 2009 Dec; 13(4):221-4.
- Benign Salivary Gland Tumors. [StatPearls. 2024] Benign Salivary Gland Tumors. Young A, Okuyemi OT. StatPearls. 2024 Jan
- Review 2021 Update on Diagnostic Markers and Translocation in Salivary Gland Tumors. [Int J Mol Sci. 2021] Review 2021 Update on Diagnostic Markers and Translocation in Salivary Gland Tumors. Meyer MT, Watermann C, Dreyer T, Ergün S, Karnati S. Int J Mol Sci. 2021 Jun 24; 22(13). Epub 2021 Jun 24.
Recent Activity
- Malignant Salivary Gland Tumors - StatPearls Malignant Salivary Gland Tumors - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Advertisement
Sclerosing mucoepidermoid carcinoma of salivary glands
- ORIGINAL ARTICLE
- Open access
- Published: 14 November 2024

Cite this article
You have full access to this open access article

- Bacem Khalele Othman 1 ,
- Martina Bradová 1 , 2 ,
- Roderick H. W. Simpson 3 ,
- Jan Laco 4 ,
- Abbas Agaimy 5 ,
- Miguel Rito 6 ,
- Stephan Ihrler 7 ,
- Petr Steiner 8 ,
- Petr Grossmann 8 ,
- Veronika Hájková 8 ,
- Gisele de Rezende 9 ,
- Montse Goma 10 ,
- Senada Koljenovic 11 , 12 ,
- Isabel Fonseca 6 ,
- Michal Michal 1 ,
- Ilmo Leivo 13 na1 &
- Alena Skalova 1 , 2 na1
325 Accesses
Explore all metrics
Sclerosing mucoepidermoid carcinoma (SMEC) of the salivary glands is a rare variant of low-grade mucoepidermoid carcinoma with scanty cellular atypia characterized by marked fibrosis/sclerosis and a rich inflammatory infiltrate. Herein, we report 25 unpublished cases of SMEC, two of them with prominent eosinophilia (2/25; 8%) and three with abundant IgG4-positive plasma cells (3/25; 12%). In our series of salivary SMEC, molecular analysis using fluorescence in situ hybridization (FISH) and/or next-generation sequencing (NGS) provided evidence of MAML2 gene rearrangement in 18 cases of the 21 analyzable cases tested (86%), while this gene locus was intact in 3 cases (14%). This study focuses on the diagnostic criteria of salivary SMEC given its challenge of abundant collagenous stroma, minimal residual neoplastic areas, and inconspicuous mucous cells. Follow-up data of our cases indicate that salivary SMECs have favorable outcomes. Molecular analysis for MAML2 gene rearrangement suggests that SMECs of salivary glands represent a rare variant of conventional low-grade MECs of salivary glands. In contrast, SMECs of the thyroid gland are genetically distinct from salivary-type thyroid MECs.
Similar content being viewed by others
Sclerosing mucoepidermoid carcinoma of the salivary glands: report of three cases with special concern to the counterpart accompanied by eosinophilia

The Challenge of “Monomorphic” Mucoepidermoid Carcinoma—Report of a Rare Case with Pure Spindle-Clear Cell Morphology
Primary Thyroid Mucoepidermoid Carcinoma (MEC) Is Clinically, Prognostically, and Molecularly Different from Sclerosing MEC with Eosinophilia: A Multicenter and Integrated Study
Avoid common mistakes on your manuscript.
Introduction
Mucoepidermoid carcinoma (MEC) is the most common malignant tumor of major and minor salivary glands characterized by mucous, intermediate, and epidermoid (squamoid) tumor cells forming cystic and solid growth patterns and usually associated with MAML2 rearrangement [ 1 ]. Diagnosis of conventional MEC is generally straightforward on histologic grounds alone. In less typical cases, the application of mucin histochemistry (mucicarmine and/or Alcian Blue/PAS/ and/or PAS-diastase stain) facilitates the identification of true intracytoplasmic mucin.
Classical MEC is in most instances an easily recognizable tumor. There is, however, a spectrum of rare histologic variants of MEC, such as oncocytic [ 2 , 3 ] , Warthin-like [ 4 , 5 ], ciliated [ 4 ], clear cell [ 6 , 7 ], pigmented [ 8 ], spindle cell [ 8 , 9 , 10 ], and mucoacinar [ 11 ] that differ from the conventional appearance. In addition, the availability of molecular testing has also made it possible to describe MEC devoid of squamoid cells using immunohistochemistry [ 12 ], monomorphic MEC with a pure spindle and clear cell pattern [ 13 ], and MEC with a unique trabecular growth pattern [ 14 ], thus expanding the histologic and immunohistochemical spectrum of MAML2 -rearranged salivary gland tumors. In such cases, reaching the correct diagnosis on histological grounds alone can be difficult. Such histological variants of MEC have now been confirmed to have characteristic molecular alterations involving the fusion transcripts CRTC1::MAML2 or CRTC3::MAML2.
Molecular testing may also be crucial for an accurate diagnosis of sclerosing mucoepidermoid carcinoma (SMEC), a rare and diagnostically challenging variant of salivary MEC. Based on previously described cases, SMEC has a tendency to form keloid-like sclerotic stroma and foci of densely packed inflammatory infiltrates situated subcapsularly or within the neoplasm itself [ 15 , 16 ]. These inflammatory infiltrates, containing eosinophils, plasma cells, and/or lymphocytes, are usually intermingled with solid neoplastic nests [ 17 , 18 , 19 ]. In cases with prevalent eosinophils in the inflammatory infiltrate, the designation of salivary sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) has been used in the literature [ 20 , 21 ].
Accurate diagnostic criteria of SMEC, however, remain controversial. Simple FISH analysis to provide evidence of MAML2 gene rearrangement may sometimes fail. Given such challenges, it is urgent to search for diagnostic histological criteria of SMEC in order to prevent misdiagnoses in difficult cases, particularly if molecular testing is not available.
It has been speculated that salivary SMECE closely resembles the thyroid counterpart, which is now included in the WHO Classification as a distinct entity [ 22 ]. Thyroid SMECE is a thyroid carcinoma composed of epidermoid and mucous cells in a background of marked stromal sclerosis with infiltration of eosinophils and lymphocytes. Genomic studies of thyroid SMECE have so far been largely uninformative, showing a notable absence of MAML2 rearrangements characteristic of mucoepidermoid carcinoma or BRAF mutations [ 23 , 24 , 25 ]. Salivary SMECE [ 20 , 21 ] has sparked a major controversy posing the question if it represents an equivalent of thyroid SMECE [ 22 ].
The aim of this study is to document 25 new cases of salivary SMEC/SMECE from consult files, including histomorphological and immunohistochemical features, molecular testing, and clinical outcomes when available. There are no reliable published criteria for the proportion of sclerosis and the composition of inflammatory infiltrates required for the diagnosis of salivary SMEC. Based on the study of our cases and review of the literature, we propose a set of major criteria for diagnosing SMEC, including keloid-like sclerosis representing more than 50% of the tumor volume and an associated heterogenous inflammatory infiltrate at the periphery and within the tumor itself. The frequency of salivary SMECs is difficult to assess, being no more than 5% of all MECs, while it may be much lower than that, as SMEC tends to be over-represented in consultation practices. In addition, we address the question of the similarity between salivary and thyroid SMEC/SMECE. Our cohort represents the largest series of salivary SMECs so far.
Materials and methods
A retrospective search in the authors’ registries was conducted to identify MECs characterized by remarkable keloid-like sclerotic stroma and dense inflammatory infiltrate. In total, 25 cases of SMEC were retrieved from the consultation files of the Tumor Registry at the Department of Pathology, Faculty of Medicine in Pilsen and Bioptic Laboratory, Ltd. in Pilsen, Czech Republic, and tumor registries of the co-authors. All cases were reviewed by the senior author (AS) and three other head and neck pathologists (RHWS, BK, and MB), and it was confirmed that they met the diagnostic criteria of SMEC, in particular, keloid-like sclerosis in more than 50% of the total tumor volume and abundant variable inflammatory infiltrate, in particular, rich on lymphocytes, plasma cells, and/or eosinophils.
Clinical features and outcomes (e.g., age, sex, site of primary tumor, follow-up period, recurrence, and distant metastasis) were recorded. Clinical information on the cases was collected from hospital records and the referring pathologists. The study was approved by the institutional review board.
Histology and immunohistochemistry
For conventional microscopy, tissues were fixed in formalin, processed routinely, embedded in paraffin (FFPE), cut, and stained with hematoxylin and eosin.
For immunohistochemistry, 4-μm-thick sections were cut from the paraffin blocks and mounted on positively charged slides (TOMO, Matsunami Glass INC, Osaka, Japan). Sections were processed on a BenchMark ULTRA (Ventana Medical Systems, Tucson, AZ), deparaffinized, and subjected to heat-induced epitope retrieval by immersion in CC1 solution (pH 8.6) at 95 °C. All primary antibodies used in this study are summarized in Table 1 . Visualization was performed using the ultraView Universal DAB Detection Kit (Roche, Tucson, AZ) and the ultraView Universal Alkaline Phosphatase Red Detection Kit (Roche, Tucson, AZ). The slides were counterstained with Mayer’s hematoxylin. Appropriate positive and negative controls were employed.
Molecular genetic studies
Next-generation sequencing.
The in-house customized version of Archer FusionPlex Solid Kit was used to construct a cDNA library for detecting fusion transcripts in 118 genes (including AKT1 , AKT3 , ALK , AR , ARHGAP26 , AXL , BCOR , BRAF , BRD3 , BRD4 , CALCA , CAMTA1 , CCNB3 , CCND1 , CD274 , CIC , CSF1 , CSF1R , DNAJB1 , EGFR , EPC1 , ERBB2 , ERBB4 , ERG , ESR1 , ESRRA , ETV1 , ETV4 , ETV5 , ETV6 , EWSR1 , FGFR1 , FGFR2 , FGFR3 , FGR , FOXO1 , FOXO4 , FUS , GLI1 , GNAS , GPI , GRB7 , HMGA2 , CHMP2A , INSR , JAK2 , JAK3 , JAZF1 , KRT20 , KRT7 , MAML2 , MAP3K3 , MAP3K8 , MAST1 , MAST2 , MEAF6 , MET , MGEA5 , MKL2 , MN1 , MSMB , MUSK , MYB , MYBL1 , MYOD1 , NCOA1 , NCOA2 , NOTCH1 , NOTCH2 , NR4A3 , NRG1 , NTRK1 , NTRK2 , NTRK3 , NUMBL , NUTM1 , PAX3 , PDGFB , PDGFRA , PDGFRB , PHF1 , PIK3CA , PKN1 , PLAG1 , PPARG , PRKACA , PRKACB , PRKCA , PRKCB , PRKD1 , PRKD2 , PRKD3 , PTH , PTPN1 , RAB7A , RAF1 , RELA , RET , ROS1 , RSPO2 , RSPO3 , SLC5A5 , SS18 , STAT6 , TAF15 , TCF12 , TERT , TFE3 , TFEB , TFG , THADA , TMPRSS2 , TTF1 , USP6 , VCP , VGLL2 , YAP1 , and YWHAE ) and point mutations in 4 genes (including BRAF , EGFR , MET , PDGFRA ). All steps were performed according to the manufacturer’s instructions, and the library was sequenced on an Illumina platform as described previously [ 26 ].
FISH and RT-PCR methodologies were described in detail in our earlier report [ 2 ]. For RT-PCR analysis, we used the primers listed in Table 2 .
TruSight oncology 500 kit (TS500)
Mutation analysis and fusion transcript detection were performed using TruSight Oncology 500 Kit (Illumina, San Diego, CA). RNA was extracted using the Maxwell RSC DNA FFPE Kit and the Maxwell RSC Instrument (Promega, Madison, WI) according to the manufacturer’s instructions and quantified using the Qubit HS RNA Assay Kit (Thermo Fisher Scientific, Waltham, MA). DNA was extracted using the QIAsymphony DSP DNA mini (Qiagen, Hilden, Germany) and quantified using the Qubit BR DNA Assay Kit (Thermo Fisher Scientific, Waltham, MA). The quality of DNA was assessed using the FFPE QC kit (Illumina) and the quality of RNA using Agilent RNA ScreenTape Assay (Agilent, Santa Clara, CA). DNA samples with Cq < 5 and RNA samples with DV200 ≥ 20 were used for further analysis. After enzymatic fragmentation of DNA with KAPAFrag Kit (KAPA Biosystems, Wilmington, MA), DNA and RNA libraries were prepared with the TruSight Oncology 500 Kit (Illumina) according to the manufacturer’s protocol. Sequencing was performed on the NovaSeq 6000 sequencer (Illumina) following the manufacturer´s recommendations. Data analysis was performed using the TruSight Oncology 500 v2.2 Local App (Illumina) as described earlier [ 27 ].
Review of the literature
The study intended to examine previously reported cases of SMEC with inflammation. We examined whether SMECs contain typical neoplastic components commonly seen in MEC and whether SMECs demonstrate charcteristic clinicopathological behavior that would require their classification separately from MEC as was proposed for SMECE of the thyroid.
The inclusion criteria for this systematic review were as follows:
All research papers must be original research articles that report clinical cases of SMEC.
All articles must be published in English.
All articles must describe the histologic appearance of SMEC in text and images.
All articles must report a confirmation of the diagnosis of MEC using histochemical, immunohistochemical, and/or molecular techniques.
The exclusion criteria were as follows:
Studies reviewing previous works without reporting any new cases
Studies that investigated non-salivary MECs
Studies that published duplicate cases
Epidemiological studies and consultation cases
Cases published by a predatory journal or non-indexed journal
We conducted an electronic search in PubMed, Science Direct, Web of Science, Scopus, Scielo, Google Scholar, EMBASE (Ovid), Europe PMC, ProQuest, Crossref, and Medline databases. The searched medical subject headings included “mucoepidermoid carcinoma”, AND “salivary gland”, AND “sclerosis*.” The search excluded “thyroid” AND/OR “breast” AND/OR “mammary” AND/OR “lung” AND/OR “pulmonary” AND/OR “pancreas*” AND/OR “skin” AND/OR “cutaneous.”
The time range was customized from 1981 to 2022. Two hundred and eighty articles were found. Duplicated articles were deleted using Mendeley software. There were 88 unique articles. After excluding non-English articles, the number of remaining articles was 53. We then screened the titles and abstracts of all of them and excluded publications not meeting the inclusion criteria. After implementing all the above criteria, the final number of remaining articles was 32 [ 15 , 16 , 17 , 18 , 19 , 20 , 21 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 ] (the process is described in Supplementary File 1 ).
Clinicopathologic features and follow-up data
The clinical and molecular characteristics of 25 patients with SMEC are summarized in Table 3 . There was a female predilection (1.5:1) with a mean age of 44 years (range 16–76 years). A parotid gland mass was the most frequent clinical presentation (23/25; 92%), while minor salivary glands of the palate and the buccal mucosa were affected in one patient each. Macroscopically, the tumors were firm, tan-white to yellow bosselated masses, and some of them had focal cystic change.
All cases were sent as consults, with the correct diagnosis made by referring pathologists in 8 cases. In 5 tumors, the diagnosis was MEC, but the feature of sclerosis as a part of the tumor was neglected in the final report. In 9 cases, the final report considers a broad spectrum of benign tumors and/or lesions, including obscure sclerosing lesions, sclerosing polycystic adenoma (SPA), sclerosing lymphadenoma, lymphoepithelial cyst, benign lymphoepithelial lesion, metaplastic Warthin tumor, and obstructive sclerosing sialadenitis. In 3 cases, another malignancy was speculated, including sclerosing mucinous cystadenocarcinoma NOS, metastatic squamous cell carcinoma, and salivary duct carcinoma, each in one case.
Follow-up data were available for 18 (72%) patients with a mean follow-up of 6.5 years (range 1–14 years). Three patients experienced local recurrences at 2, 4, and 4 years after primary surgery, respectively. One patient had a single periglandular lymph node metastasis and refused radiotherapy. Nevertheless, there was no evidence of disease 4 years after primary diagnosis. None of the 18 patients revealed distant metastasis (Table 3 ).
Histological and immunohistochemical findings
Microscopically, the cases of SMECs without prominent infiltration by eosinophils were well circumscribed and only partially encapsulated by a hyalinized pseudocapsule. At the periphery of the lesion, there was a localized or circumscribed zone of tumor-associated lymphoid tissue (TALP) with occasional lymphoid follicles and even germinal centers (Fig. 1 A). TALP was composed of a variable population of inflammatory cells including small and intermediate-sized lymphocytes, plasma cells, histiocytes, and a few neutrophils and/or mast cells. The lymphoid tissue was incorporated in the form of single-cell strips or elongated nests within the lesion and dissected collagen bundles with evidence of retraction artifact at their periphery (Fig. 1 B). The tumors were composed of a centrally located paucicellular zone of hyalinized collagen in a scar-like fashion including few fibroblasts (Fig. 1 C) and entrapped inflammatory and/or lesional cells which were immunostained by antibodies to cytokeratins AE1/AE3 and CK7 and to p40 and/or p63 (Fig. 1 D, E ). The stromal component was always pronounced and comprised more than 50% of the total tumor volume and revealed fibrohyaline sclerotic features resembling a storiform collagenoma (Fig. 1 C). Tumor cells were less prominent representing 5–20% of the total tumor volume, and they were dispersed in single cell or file fashion between the collagen bundles. Occasionally, the tumor cells grew in variably sized nests or irregular cystic spaces with predominant intermediate cell and mucous cell differentiation (Fig. 1 A, F ). The tumor cells were spindle-shaped to oval, sharply demarcated, with mild pleomorphism, distinct eosinophilic nucleoli, and a moderate amount of eosinophilic to clear cytoplasm. The mucous cells were distinguished by a cytoplasm distended by a pale mucous vacuole displaying positivity for periodic-acid Schiff and/or mucicarmine. Some cystic structures were compromised, and their mucous content was spilled out into the stroma.

SMECs were well circumscribed and only partially encapsulated by hyalinized pseudocapsule with tumor-associated lymphoid tissue at the periphery including lymphatic follicles with germinal centers and displaying a paucicellular zone of hyalinized collagen in the center A . The inflammatory component was mixed, predominantly composed of lymphocytes and plasma cells with only a few cells of acute inflammation (neutrophils/mast cells) B . The stromal component was always pronounced and revealed fibrohyaline sclerotic features resembling storiform collagenoma with tumor cells entrapped in a single fashion or as small clusters of cells C , D . Higher magnification of scattered tumor cells dispersed in collagen bundles D and highlighted by AE1/3 E on the left of both pictures with entrapped nerve. Occasionally there were characteristic structures of MEC arranged in cystic spaces with intermediate and mucoid cell differentiation F
There was only minimal perineural invasion (PNI) in two cases, and occasionally, nerves were entrapped in the hyaline sclerotic stroma (Fig. 1 D, E ) or at the periphery of the lesion.
Lymphovascular invasion (LVI), necrosis, anaplasia, or prominent mitotic activity were not detected. Proliferative activity was low with mitotic index (MIB1) ranging between 5 and 10%. In 4 cases, the tumors contained foci of a giant cell reaction with multinucleated histiocytes (not shown).
Of these 23 SMECs without eosinophils, 3 cases showed IgG4-positive plasma cells in addition to the classic features of this entity. Immunohistochemical staining shows IgG + and IgG4 + plasma cells in three cases (Fig. 2 A, B ). The distribution of these cells was non-specifically predominant at the peripheral inflammatory infiltrates with elevated IgG4 + /IgG + ratios (23–33%). However, peri-tumoral areas demonstrate subsidiary cell densities and IgG4 + /IgG + ratios (14–18%). There was no intratumoral detection of either IgG4 + or IgG + cells (Table 4 ). This may suggest a reactive inflammatory process with increased IgG4-positive cells, yet not meeting strict IgG4-related disease criteria. No other microscopic features of IgG4-related disease, i.e., storiform fibrosis or obliterative phlebitis, were found. None of the patients had systemic IgG4-related disease.

Three cases with typical SMEC-histology characterized by entrapped lesional structures in dense hyalinosclerotic stroma with intense inflammatory (mainly lymphoplasmacytic) infiltrate A showed an increased number of IgG4-positive plasma cells B
Two SMECs showed a predominant admixture of eosinophils in the inflammatory component (SMECE). These tumors were well circumscribed and almost ovoid lesions with a very thin fibrous pseudocapsule at the periphery (Fig. 3 A). The cellularity of the two SMECE cases was high. The neoplastic cells were surrounded and permeated by a dense lymphoplasmacytic infiltrate with an abundant admixture of eosinophils and focal accumulations of chronic inflammatory cells, in particular, lymphocytes in lymphoid follicles with germinal centers (Fig. 3 B), and no IgG4-positive plasma cells. The neoplastic cells were haphazardly dispersed throughout the lesion arranged in variably sized nests, thin strands, and anastomosing cords (Fig. 3 C). Occasionally, the neoplastic cells created irregular cystic spaces containing PAS-positive amorphous material or eosinophils (Fig. 3 D). The neoplastic cells were large and polygonal, almost ganglion-like in morphology, and their nuclei were round to oval with prominent single nucleoli and vesicular chromatin, and the cytoplasm was eosinophilic or vacuolized. Areas of squamoid cells with evidence of abrupt keratinization were present focally (Fig. 3 D). The inflammatory cell and neoplastic cell components represented 50% and 30% of the tumor volume, respectively. The stromal component was less prominent when compared to SMEC, and it represented about 20% of the tumor volume. The stroma consisted of thin hyalinized membranes and/or fibrous septa or large fibrous fascicles that surrounded the neoplastic cells (Fig. 3 E) positive for p63 (Fig. 3 F) and negative for SOX10. In one case, there was PNI (Fig. 3 B). None of the SMECs showed LVI, necrosis, or an increased mitotic count. One particular aspect of these tumors is that none of the cases showed both abundant eosinophils and IgG4-positive plasma cells together.

SMECEs were highly cellular with the accumulation of chronic inflammatory cells, in particular lymphocytes that create lymphatic follicles with germinal centers A . The lesions were arranged in variably sized nests, thin strands, and anastomosing cords of large polygonal ganglion-like cells; their nuclei were round to oval with prominent nucleoli, vesicular chromatin, and eosinophilic or vacuolized cytoplasm B with neural involvement (arrow). Cystic spaces with squamoid cells and evidence of abrupt keratinization C and sometimes with multiple eosinophils in their lumen D . The stromal component consisted of thin hyalinized membranes and/or fibrous septa that surrounded tumor cells with a retraction phenomenon at their periphery. Occasionally they create larger fibrous fascicles E . Tumor cells were positive for p63 F
Established grading systems given for MECs were used in all 25 cases [ 1 ]. There were 22 and 3 cases graded low- and intermediate-grade, respectively, using the AFIP system, while using the Brandwein modified system, 19, 2, and 4 cases were graded low, intermediate, and high, respectively.
Molecular profile and fluorescence in situ hybridization
All analyzable cases were studied for MAML2 gene break or gene fusion using FISH (21/25), RT-PCR (8/25), or NGS (13/25), respectively. Rearrangement of the MAML2 gene was detected in 18 cases of the 21 analyzable cases tested (86%) by FISH, while this gene locus was intact in 3 cases (14%). In two cases, FISH analysis was not performed (due to lack of tissue material), and two tumors were not analyzable. In addition, six cases of SMECs showed CRTC1::MAML2 fusion transcript using RT-PCR and three with TS500 NGS methodology, respectively. The used NGS panels investigated cover the fusions and mutations of all genes commonly reported in salivary gland tumors. Only Case#2 showed PIK3CA , NF , TCF7L2 , and PTPRD mutations. Molecular findings in all 25 cases are summarized in Table 3 .
Thirty-three articles have reported forty-seven cases of SMEC containing inflammatory infiltrates with one or more cell types (e.g., eosinophils, neutrophils, lymphocytes, and/or plasma cells). Epidemiologically, the reporting countries were the USA (31.9% of the cases), Japan (29.8%), India (14.9%), South Korea (4.3%), and the UK (4.3%). A single case each was contributed from South Africa, Brazil, Canada, Hong Kong, Iran, Ireland, and Romania. Two-thirds of the published cases came from the USA and Japan. See Supplementary file 2 for information on the included cases.
Major salivary glands were the preferable sites of origin of SMEC, including parotid (61.7%), submandibular (14.9%), and sublingual glands (4.3%). In minor salivary glands, SMECs were localized in the palate (6.4%), the parapharyngeal space (4.3%), the retromolar area (4.3%), and the upper lip (4.3%). Females were affected more often than males (2.36: 1). The mean age of patients was 51 years (range 16–81 years). Fifty-seven percent of the reported tumors were small in size (< 2 cm; T1). The remaining cases were medium size (2–4 cm; T2). Many articles reported radiographic measurements of the lesion or macroscopic measures of the gross resection, but radiological information was missing from many case studies (Supplementary file 2 ).
Low-grade histology was demonstrated in 75% of the reported SMEC cases, while 17% were intermediate-grade, 4% were high-grade, and 4% were not evaluated. In the inflammatory infiltrates, the predominant cells were eosinophils (34%), followed by plasma cells (19%), lymphoplasmacytic cells (17%), neutrophils (13%), lymphocytes (9%), immunoblasts (2%), foamy histiocytes (2%), and mast cells (2%).
The presence of mucin spillage into the stroma forming mucin pools and surrounded by inflammatory infiltrates was observed in all cases. Stromal fibrosis, hyalinization, and sclerosis were also pathognomonic. Other histologic features included cell keratinization (10.6%) [ 34 , 43 ], eosinophil abscess formation (10.6%) [ 16 , 47 ], lymph node metastasis (6.4%) [ 31 ], tumor-associated lymphoid proliferation (TALP) (6.4%) [ 18 ], perineural invasion (4.3%) [ 29 ], calcification (4.3%) [ 17 , 30 ], necrosis (2.1%), apocrine differentiation (hobnail pattern) (2.1%) [ 47 ], spindle cell areas (2.1%) [ 19 ], pigmentation and dendritic melanocytes (2.1%) [ 19 ], and sebaceous differentiation (2.1%) [ 47 ]. In 14.9% of the SMEC cases, the inflammatory infiltrates contained also IgG4-positive plasma cells, while storiform fibrosis and obliterative phlebitis were, however, absent [ 15 , 16 , 20 , 40 ].
Only ten cases described in the literature were molecularly tested for MAML2 gene break and/or fusion using FISH or RT-PCR. MAML2 gene rearrangement was reported in four of these cases (4/10; 40%) [ 15 , 19 , 38 , 49 ]. Such a low percentage of MAML2 fusion may be attributed to technical challenges in a tumor with a low number of neoplastic cells.
Mucoepidermoid carcinoma (MEC) is the most common salivary malignancy, and in most cases, it is an easily recognizable tumor. In contrast, the sclerosing variant of salivary MEC (SMEC) with or without eosinophilia is a rare and enigmatic salivary gland neoplasm with a broad differential diagnosis, which presents difficulties in correct categorization. SMEC has a predilection for parotid glands in young/middle-aged patients. It is more commonly encountered in women and usually manifests as a painless slowly growing tumor. Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) is an exceptionally rare low-grade variant of thyroid carcinomas. Since its first description in 1991 [ 53 ], fewer than 100 cases have been reported [ 23 , 24 , 25 , 54 ]. The disease presents more frequently in females, on average in the 5th decade. Although some similarities to salivary gland MEC are observed, the current concept is that SMECE of the thyroid is distinct from salivary-type MEC [ 23 , 55 ]. Histologically, thyroid SMECE shows anastomosing cords and narrow strands of neoplastic mucocytes and epidermoid cells with keratinization and intercellular bridges infiltrating a sclerotic stroma. Thus, thyroid SMECE is morphologically similar but still different from salivary gland SMEC. Unlike the latter, mature squamous differentiation (i.e., keratinization) is a common and even defining feature of thyroid SMECE. Furthermore, the intermediate-type cells that define salivary gland MECs are not present in thyroid SMECE. The stroma in thyroid SMECE reveals an inflammatory background in which prominent eosinophils, lymphocytes, and plasma cells are the main inflammatory cells. Molecular investigation has established that thyroid SMECE is negative for MAML2 rearrangement [ 23 ]. Five cases successfully tested by NGS (ThyroSeq v.2 assay and solid tumor fusion panel) were also negative for mutations and translocations commonly involved in thyroid carcinogenesis [ 23 ]. Consequently, thyroid SMECE is not considered to be a part of the spectrum of MEC or papillary thyroid carcinoma, but it is a distinctive entity [ 22 , 23 ]. In contrast, in our series of salivary SMEC, FISH was more successful in detecting MAML2 gene rearrangement, and therefore, salivary SMEC seems to represent a rare variant of conventional MEC with the same molecular underpinnings. Although the majority of the reported thyroid SMECE cases had an indolent course [ 56 ], aggressive high-grade SMECE with extension to extrathyroid tissues and distant spread have been reported [ 57 ]. In contrast, salivary SMEC appears to have a generally favorable outcome.
As shown by the original diagnoses in the present study, the diagnosis of salivary SMEC is challenging because of a broad spectrum of potential diagnostic pitfalls including non-neoplastic inflammatory lesions and benign and/or malignant tumors with prominent hyaline sclerosis. Conventional salivary MEC may contain interspersed fibrous areas usually separating cell nests, while SMECs, with or without inflammatory cells, display prominent sclerosis and keloid-like hyalinized sclerotic stromal foci that may even efface most of the neoplastic proliferation. In our cohort, keloid-like hyaline sclerosis amounted to at least 50% or more of the total tumor volume (mean 63%), regardless of the amount and composition of the inflammatory cells. We assume that this dense sclerosis interferes with the optical properties of the signals the neoplastic cells convey in FISH. Therefore, the greater the volume of the sclerotic area is, the less likely it is that a MAML2 translocation/rearrangement can be proven. Similarly, NGS analysis might be unsuccessful due to the low number of neoplastic cells in the specimen. This poses additional challenges to the diagnosis of SMEC of salivary glands. Nevertheless, based on above mentioned histomorphological criteria, even the cases either negative or not analyzable for MAML2 gene break could be classified as highly suspicious for SMEC.
In the differential diagnosis of SMEC, sclerosing polycystic adenoma of salivary glands (SPA) with extensive hyaline sclerotic areas must be taken into consideration [ 58 , 59 ]. SPA is a benign, often sclerotic tumor that harbors genetic alterations in the PI3K pathway [ 58 , 59 ] and may show areas of mild to severe dysplasia [ 59 ]. The hallmark of SPA is the presence of acini containing large brightly eosinophilic cytoplasmic granules/globules. Although SPA is often associated with dysplasia and most likely represents a precursor lesion of malignancy, it is benign and does not harbor the CRTC1 :: MAML2 gene fusion [ 59 ].
As SMEC cases with abundant IgG4-positive plasma cells were reported [ 20 , 40 ], IgG4-related disease (IgG4 sialadenitis) enters the differential diagnosis. In our series, we have identified three cases with prominent IgG4-positive plasma cells, but there were not any other features of IgG4-related disease, such as storiform fibrosis or obliterative phlebitis. First, IgG4 sialadenitis typically affects the submandibular glands (usually bilaterally), whereas SMEC most frequently manifests in the parotid gland and as a unilateral tumor. Second, characteristic features of IgG4-related disease, i.e., storiform fibrosis and obliterative phlebitis, are typically not observed in SMEC. Third, any degree of cellular atypia on the fibro-sclero-inflammatory background in salivary glands should raise a suspicion of malignancy (SMEC) as it is typically not seen in IgG4 sialadenitis. More precisely, the presence of “cellular infiltrates suspicious for malignancy” is one of the exclusion criteria for IgG4-related disease [ 60 ]. Molecular testing for MAML2 rearrangement may be of great value in difficult cases. Previously reported features in SMEC such as IgG4-related inflammation, Küttner’s disease, and tumor-associated lymphoid tissue (TALP) cannot be considered pathognomonic for salivary SMEC, because these findings have also been reported in other salivary gland lesions [ 61 ]. MEC with inflammation (including TALP), but without sclerosis, is a common finding, but this does not represent SMEC by definition [ 62 ].
The current grading systems for mucoepidermoid carcinoma (MEC) are either quantitative (based on scoring) including AFIP [ 63 ] and Brandwein [ 64 ] or qualitative including a 2-tiered system developed by Xu et al. [ 65 ]. Especially when dealing with cases featuring extensive sclerosis, there are discrepancies in how these grading systems assess such cases highlight their deficiencies in offering reliable prognostic information. If the new Memorial Sloan Kettering Cancer Center (MSK) scoring was applied [ 65 ], many SMEC cases would be of high grade. Follow-up data from our cases and from the literature indicate, however, that salivary SMECs have favorable outcomes. Established grading systems given for MECs [ 1 ] are not reliably applicable to SMEC.
We reviewed all previously reported cases of salivary SMEC and summarized their shared histologic, molecular, and immunohistochemical findings. In addition, here, we report 25 unpublished cases of SMEC from the consult files of the authors, which is the largest cohort so far. Salivary SMEC is characterized by the formation of keloid-like sclerotic stroma amounting to more than 50% of the tumor volume accompanied by foci of densely packed inflammatory infiltrates subcapsularly or within the neoplasm itself. Such inflammatory infiltrates (eosinophils, plasma cells, or lymphocytes) are usually intermingled with solid nests of neoplastic cells. In contrast to thyroid SMECE which is a distinct entity arising in the background of fibrosing Hashimoto thyroiditis and lacking MAML2 gene alterations, salivary SMEC is a rare variant of MEC characterized by MAML2 gene break and/or CTRC1/CRTC3::MAML2 gene fusion.
Data availability
Data supporting the findings of this study are available within the article. The complete datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
WHO Classification of Tumours editorial board. Head and neck tumours. 5 th ed. Lyon (France): International Agency for Research on Cancer; 2022. (WHO Classification of Tumours series, ; vol. 9).; 2022. Available at: https://publications.iarc.fr/
Skálová A, Agaimy A, Stanowska O, Baneckova M, Ptáková N, Ardighieri L et al (2020) Molecular profiling of salivary oncocytic mucoepidermoid carcinomas helps to resolve differential diagnostic dilemma with low-grade oncocytic lesions. Am J Surg Pathol 44(12):1612–1622
Article PubMed Google Scholar
Weinreb I, Seethala RR, Perez-Ordoñez B et al (2009) Oncocytic mucoepidermoid carcinoma: clinicopathologic description in a series of 12 cases. Am J Surg Pathol 33:409–416
Bishop JA, Cowan ML, Shum CH, Westra WH (2018) MAML2 Rearrangements in variant forms of mucoepidermoid carcinoma: ancillary diagnostic testing for the ciliated and Warthin-like variants. Am J Surg Pathol 42(1):130–136
Article PubMed PubMed Central Google Scholar
Ishibashi K, Ito Y, Masaki A, Fujii K, Beppu S, Sakakibara T et al (2015) Warthin-like mucoepidermoid carcinoma: a combined study of fluorescence in situ hybridization and whole-slide imaging. Am J Surg Pathol 39(11):1479–1487
Tajima S, Namiki I, Koda K (2017) A clear cell variant of mucoepidermoid carcinoma harboring CRTC1-MAML2 fusion gene found in buccal mucosa: report of a case showing a large clear cell component and lacking typical epidermoid cells and intermediate cells. Med Mol Morphol 50(2):117–121
Article CAS PubMed Google Scholar
Skalova A, Leivo I, Hellquist H, Simpson RHW, Vander Poorten V, Willems SM et al (2022) Clear cell neoplasms of salivary glands: a diagnostic challenge. Adv Anat Pathol 29(4):217–226
Oide T, Hiroshima K, Takahashi Y et al (2017) Mucoepidermoid carcinoma with extensive spindled morphology and melanocytic marker expression. Hum Pathol 67:181–186
Goh GH, Lim CM, Vanacek T, Michal M, Petersson F (2017) Spindle cell mucoepidermoid carcinoma of the palatine tonsil with CRTC1-MAML2 fusion transcript: report of a rare case in a 17-year-old boy and a review of the literature. Int J Surg Pathol 25(8):705–710
Ide F, Mishima K, Saito I (2008) Mucoepidermoid carcinoma with spindle cell change: a low-grade lesion potentially mistaken for sarcomatoid dedifferentiation. Head Neck Pathol 2:227–230
Bundele M, Weinreb I, Xu B et al (2021) Mucoacinar carcinoma: a rare variant of mucoepidermoid carcinoma. Am J Surg Pathol 45:1028–1037
Bishop JA, Thompson LDR, Siegele B, Gagan J, Mansour M, Chernock RD et al (2023) Mucoepidermoid carcinoma may be devoid of squamoid cells by immunohistochemistry: expanding the histologic and immunohistochemical spectrum of MAML2-rearranged salivary gland tumours. Histopathology 82(2):305–313
Qu X, Chew EJC, Selvarajan S, Wu B, Agaimy A, Petersson F (2023) The challenge of “monomorphic” mucoepidermoid carcinoma-report of a rare case with pure spindle-clear cell morphology. Head Neck Pathol 17(3):864–870
Ahn B, Choi SH, Kim D, Kim D, Cho KJ (2023) Salivary gland neoplasms with a unique trabecular histology and MAML2 translocation: a trabecular variant of a mucoepidermoid carcinoma. Am J Surg Pathol 47(10):1085–1095
Yabuki K, Matsuyama A, Shiba E et al (2018) Sclerosing mucoepidermoid carcinoma in the parotid gland with CRTC1-MAML2 fusion: a case report. Int J Surg Pathol 26:250–255
Tasaki T, Matsuyama A, Tabata T, Suzuki H, Yamada S, Sasaguri Y, Hisaoka M (2013) Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary gland: case report and review of the literature. Pathol Int 63(2):125–131
Heptinstall L, Carroll C, Siddiqi J et al (2017) Sclerosing mucoepidermoid carcinoma of the submandibular gland presenting as chronic sialadenitis: a case report and review of literature. Head Neck Pathol 11:506–512
Article CAS PubMed PubMed Central Google Scholar
Harada H, Toyozumi Y, Sasaguri T et al (2021) Sclerosing mucoepidermoid carcinoma of the salivary glands: report of three cases with special concern to the counterpart accompanied by eosinophilia. Med Mol Morphol 54:265–274
Harada H, Takeda M, Kohno Y, et al (2021) Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary glands: two additional cases not harboring MAML2 gene rearrangement. Hum Pathol Case Reports 25
Mendelson AA, Al-macki K, Chauvin P et al (2013) Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary gland: case report and review of the literature. Pathol Int 63:125–131
Article Google Scholar
Urano M, Abe M, Horibe Y et al (2002) Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary glands. Pathol Res Pract 198:305–310
Sobrinho-Simões et al (2017) WHO Classification of Tumours of endocrine organs. In: Lloyd RV, Osamura RY, Klöppel G RJ, ed. WHO Classification of Tumours of endocrine glands. 4th Ed. Lyon:IARC. 4 th. Lyon: IARC; 2017:119–120
Shah AA, La Fortune K, Miller C et al (2017) Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Mod Pathol 30:329–339
Wiles AB, Kraft AO, Mueller SM, Powers CN (2019) Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: case report of a rare lesion with novel genetic mutation. Diagn Cytopathol 47(6):589–593
Agaimy A, Tögel L, Stoehr R, Meidenbauer N, Semrau S, Hartmann A et al (2021) NSD3-NUTM1 -rearranged carcinoma of the median neck/thyroid bed developing after recent thyroidectomy for sclerosing mucoepidermoid carcinoma with eosinophilia: report of an extraordinary case. Virchows Arch 479(6):1095–1099
Skálová A, Ptáková N, Santana T et al (2019) NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is “intraductal” correct? Am J Surg Pathol 43:1303–1313
Skálová A, Taheri T, Bradová M, Vaněček T, Franchi A, Slouka D, et al. SMARCB1-deficient sinonasal adenocarcinoma: a rare variant of SWI/SNF-deficient malignancy often misclassified as high-grade non-intestinal-type sinonasal adenocarcinoma or myoepithelial carcinoma. Virchows Arch. 2023 Dec 12. https://doi.org/10.1007/s00428-023-03650-2
Chan JKC, Saw D (1987) Sclerosing mucoepidermoid tumour of the parotid gland: report of a case. Histopathology 11:203–207
Muller S, Barnes L, Goodurn WJ (1997) Sclerosing mucoepidermoid carcinoma of the parotid. Oral Surg Oral Med Oral Pathol Oral Radiol 83:685–690
Sinha SK, Keogh IJ, Russell JD et al (1999) Sclerosing mucoepidermoid carcinoma of minor salivary glands: a case report. Histopathology 35:283–284
Fadare O, Hileeto D, Gruddin YL et al (2004) Sclerosing mucoepidermoid carcinoma of the parotid gland. Arch Pathol Lab Med 128:1046–1049
Ide F, Obara K, Enatsu K et al (2005) Sclerosing mucoepidermoid carcinoma of the oral cavity. J Oral Pathol Med 34:187–189
Heavner SB, Shah RB, Moyer JS (2006) Sclerosing mucoepidermoid carcinoma of the parotid gland. Eur Arch Oto-Rhino-Laryngology 263:955–959
Kim H, Lee J-H, Lee ES et al (2007) Sclerosing mucoepidermoid carcinoma of the parotid gland - a case report. Korean J Pathol 41:193–197
Google Scholar
Veras EFT, Sturgis E, Luna MA (2007) Sclerosing mucoepidermoid carcinoma of the salivary glands. Ann Diagn Pathol 11:407–412
Aguiar MC, Bernardes VF, Cardoso SV et al (2008) A rare case of sclerosing mucoepidermoid carcinoma arising in minor salivary glands with immunohistochemical evaluation. Minerva Stomatol 57:453–457
CAS PubMed Google Scholar
Shinhar SY (2009) Sclerosing mucoepidermoid carcinoma of the parotid gland: Case report. Ear Nose Throat J 88
Kasai T, Takeda M, Enomoto Y et al (2011) Molecular detection of MECT1-MAML2 fusion gene in mucoepidermoid carcinoma with ordinary and variant histology: a study using archival paraffin embedded tissue. J Nara Med Assoc 62:69–79
CAS Google Scholar
Mardi K, Madan S (2012) Sclerosing mucoepidermoid carcinoma of the submandibular gland: report of two rare cases. Clin Cancer Investig J 1:86
Tian W, Yakirevich E, Matoso A et al (2012) IgG4+ plasma cells in sclerosing variant of mucoepidermoid carcinoma. Am J Surg Pathol 36:973–979
Boaz K, Mehta KK, Natarajan S et al (2013) Palatal swelling in a patient suffering from filariasis. J Clin Diagnostic Res 7:2651–2654
Bhat K, Pandey B, Shetty P, et al (2014) Sclerosing mucoepidermoid carcinoma: a unique case. Sultan Qaboos Univ Med J 14
MewaKinoo S, Maharaj K, Singh B, Govender M, Ramdial PK (2014) Primary esophageal sclerosing mucoepidermoid carcinoma with “tissue eosinophilia.” World J Gastroenterol 20(22):7055–7060
Lohiya PG, Chaudhary MS, Patil S et al (2015) Sclerosing mucoepidermoid carcinoma of minor salivary gland. Contemp Clin Dent 5:564–568
Kobayashi Y, Satoh K, Aizawa T, et al (2015) Local recurrence of sclerosing mucoepidermoid carcinoma with eosinophilia in the upper lip: a case report. J Med Case Rep 9
Bidari-Zerehpoosh F, Naghibzadeh B, Jamali E et al (2016) Sclerosing mucoepidermoid carcinoma of the parotid gland. Iran J Otorhinolaryngol 28:281–285
PubMed PubMed Central Google Scholar
Gherghina FL, Camen A, Munteanu MC et al (2016) Parotid sclerosing mucoepidermoid carcinoma: a case report and immunohistochemical study. Rom J Morphol Embryol 57:1107–1116
PubMed Google Scholar
Lee DH, Kim JH, Lee JK et al (2017) Sclerosing mucoepidermoid carcinoma of the sublingual gland. Eur Ann Otorhinolaryngol Head Neck Dis 134:355–356
Sato K, Akiba J, Nakamura K et al (2017) Mucoepidermoid carcinoma of the sublingual gland harboring a translocation of the MAML2 gene: a case report. Oncol Lett 14:2970–2974
Devi A, Narwal A, Kamboj M et al (2018) A mismatch of tumor grade and biologic behaviour in a rare case of sclerosing mucoepidermoid carcinoma of parotid - with review of literature. J Exp Ther Oncol 12:307–315
Fujioka H, Koike T, Imamura T, et al (2020) Mucoepidermoid carcinoma of parotid gland and membranous nephropathy - differentiation between sclerosing mucoepidermoid carcinoma with eosinophilia and Kimura’s disease. BMC Nephrol 21
Rasul U, Bradish T, Bashir MT, et al (2020) Sclerosing variant of mucoepidermoid carcinoma: a diagnostic challenge. BMJ Case Rep 13
Chan JK, Albores-Saavedra J, Battifora H, Carcangiu ML, Rosai J (1991) Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto’s thyroiditis. Am J Surg Pathol 15:438–448
Baloch ZW, Solomon AC, LiVolsi VA (2000) Primary mucoepidermoid carcinoma and sclerosingmucoepidermoid carcinoma with eosinophilia of the thyroid gland: a report of nine cases. Mod Pathol 13:802–807
Baloch ZW, LiVolsi VA (2018) Special types of thyroid carcinoma. Histopathology 72:40–52
Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O (2022) Overview of the 2022 WHO Classification of thyroid neoplasms. Endocr Pathol 33(1):27–63
Shehadeh NJ, Vernick J, Lonardo F, Madan SK, Jacobs JR, Yoo GH, Kim HE, Ensley JF (2004) Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: a case report and review of the literature. Am J Otolaryngol 25(1):48–53
Bishop JA (2021) Thompson LDR. Sclerosing polycystic adenoma. Surg Pathol Clin 14:17–24
Skálová A, Baněčková M, Laco J, Di Palma S, Agaimy A, Ptáková N et al (2022) Sclerosing polycystic adenoma of salivary glands: a novel neoplasm characterized by PI3K-AKT pathway alterations-new insights into a challenging entity. Am J Surg Pathol 46(2):268–280
Wallace ZS, Naden RP, Chari S, et al (2020) American College of Rheumatology/European League against rheumatism IgG4-related disease classification criteria working group. The 2019 American College of Rheumatology/European League against rheumatism classification criteria for IgG4-related disease. Arthritis Rheumatol 72(1):7–19
Laco J, Ryska A, Celakovsky P et al (2011) Chronic sclerosing sialadenitis as one of the immunoglobulin G4-related diseases: a clinicopathological study of six cases from Central Europe. Histopathology 58:1157–1163
Laforga JB (2020) Mucoepidermoid carcinoma with inflammatory lymphocytic background: a potential misinterpretation. Diagn Cytopathol 48(1):93–95
Auclair PL, Goode RK, Ellis GL (1992) Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 69(8):2021–30
Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE (2001) Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 25(7):835–845
Xu B, Alzumaili B, Furlan KC, Martinez GH, Cohen M, Ganly I, Ghossein RA, Katabi N (2023) Critical appraisal of histologic grading for mucoepidermoid carcinoma of salivary gland: is an objective prognostic 2-tiered grading system possible? Am J Surg Pathol 47(11):1219–1229
Download references
Open access publishing supported by the National Technical Library in Prague. This study was supported by study grant SVV 260652 from the Ministry of Education of the Czech Republic (NK), the Cooperation Program—research area SURG from the Charles University, Czech Republic (MB, AS), the project National Institute for Cancer Research—NICR (Programme EXCELES, ID Project No. LX22NPO5102)—funded by the European Union—Next Generation EU (AS, MB), and Turku University Hospital Fund, Maritza and Reino Salonen Foundation, and the Finnish Cancer Society, Finland (IL).
Author information
Ilmo Leivo and Alena Skalova are contributed equaly to this work.
Authors and Affiliations
Department of Pathology, Faculty of Medicine in Pilsen, Charles University, Pilsen, Czech Republic
Bacem Khalele Othman, Martina Bradová, Michal Michal & Alena Skalova
Bioptic Laboratory, Ltd, Pilsen, Czech Republic
Martina Bradová & Alena Skalova
Department of Pathology, University of Calgary, Calgary, Alberta, Canada
Roderick H. W. Simpson
The Fingerland Department of Pathology, Faculty of Medicine, Charles University, Hradec Králové and University Hospital Hradec Králové, Hradec Králové, Czech Republic
Institute of Pathology, University Hospital Erlangen, Friedrich‐Alexander University Erlangen‐Nürnberg (FAU), Comprehensive Cancer Center (CCC) Erlangen-EMN, Erlangen, Germany
Abbas Agaimy
Department of Pathology, Faculdade de Medicina, Instituto Português de Oncologia de Lisboa Francisco Gentil & Institute of Pathology, Universidade de Lisboa, Lisbon, Portugal
Miguel Rito & Isabel Fonseca
DERMPATH, Munich, Germany
Stephan Ihrler
Molecular and Genetic Laboratory, Bioptic Laboratory, Ltd, Pilsen, Czech Republic
Petr Steiner, Petr Grossmann & Veronika Hájková
Department of Anatomic Histopathology and Cytogenetics, Department of Laboratory Medicine, Niguarda Cancer Center, Milan, Italy
Gisele de Rezende
Department of Pathology, Hospital Universitari de Bellvitge, Barcelona, Spain
Montse Goma
Department of Pathology, Antwerp University Hospital, University of Antwerp, Antwerp, Belgium
Senada Koljenovic
Department of Pathology, Erasmus University Medical Center, Rotterdam, The Netherlands
Institute of Biomedicine, Pathology, University of Turku and Department of Pathology, Turku University Hospital, Turku, Finland
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. BK, MB, and AS: material preparation, data collection, and analysis. The first draft of the manuscript was written by Bacem Khalele and Alena Skálová. AS, AA, RHWS, IL, and MM: conception and design of the work, acquisition, analysis and interpretation of data, drafting the MS, and revising it critically for important intellectual content and scientific integrity. PS, PG, and VH performance and interpretation of molecular genetic analysis and revising it critically for important intellectual content and scientific integrity. JL, MR, AA, SI, GR, MG, and SK: providing the case and reading and revising the MS critically for important intellectual content and scientific integrity. All authors have read and approved the final manuscript. This manuscript has two senior authors, AS and IL.
Corresponding author
Correspondence to Alena Skalova .
Ethics declarations
Ethics approval.
The study was approved by the institutional review board of the Faculty of Medicine in Pilsen, Charles University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
No patient consent was required for this study.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. AA is the Editor-in-Chief of Virchows Archiv. AS serves on the Editorial Board of Virchows Archiv.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 25 KB)
Supplementary file2 (docx 30 kb), rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Othman, B.K., Bradová, M., Simpson, R.H.W. et al. Sclerosing mucoepidermoid carcinoma of salivary glands. Virchows Arch (2024). https://doi.org/10.1007/s00428-024-03970-x
Download citation
Received : 26 August 2024
Revised : 27 October 2024
Accepted : 07 November 2024
Published : 14 November 2024
DOI : https://doi.org/10.1007/s00428-024-03970-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sclerosing mucoepidermoid carcinoma
- Salivary gland
- MAML2 rearrangement
- Tissue eosinophilia
- Keloid-like stromal fibrosis
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Abstract. Salivary gland cancers (SGCs), categorized as head and neck cancers (HNCs), constitute about 6% of head and neck cancer diagnoses based on estimate by American Head and Neck Society. Salivary gland tumors originate from different glandular cell types and are thus morphologically diverse. These tumors arise from any of the three major ...
Squamous cell carcinoma (SCC) is one of the most common cancers of the oral cavity, but the salivary glands are rarely affected by this malignancy. The exact frequency of primary tumors in salivary glands remains unknown, as cancer cells from skin squamous cell carcinoma might use glandular tissue as a secondary site for metastasis.
Notably, cluster 0 salivary adenoid cystic carcinoma appears to be common, underscoring its predominant role in salivary gland cancer research. Additionally, we also noticed a significant number of manuscripts focusing on the salivary gland in relation to other types of cancer, such as thyroid cancer and prostate cancer.
EPIDEMIOLOGY OF SALIVARY GLAND MALIGNANCIES. Salivary gland malignancies are uncommon, comprising approximately 6%–8% of all head and neck cancers. 1 Although wide variations in incidence have been reported around the world, depending on the population studied, salivary gland malignancies occur at an incidence of approximately 1.1 cases per 100,000 individuals in the United States. 2 At ...
1. Introduction. Salivary gland malignancies (SGMs) are rare malignancies in the head and neck region representing fewer than 5% of the newly diagnosed head and neck neoplasms [1, 2]. The WHO describes almost 40 different tumors in the salivary gland region of which the most frequent malignant SGMs are muco-epidermoid carcinoma (MEC) [3, 4, 5 ...
Salivary gland carcinoma (SGC) is a rare tumor and represents ~6% of head and neck cancers (1). Malignant tumors of the salivary glands constitute a heterogeneous group of neoplasms that vary depending on the histology and their anatomical location. According to the 2017 WHO Classification, there are 24 malignant histological subtypes (2).
Salivary gland tumors are a rare group of complex, heterogeneous histologies located in the parotid, submandibular, sublingual, and minor salivary glands of the upper aerodigestive tract. The wide variety of tumor etiology, microscopic histology, growth patterns, and tumor characteristics can make diagnosis and treatment challenging for clinicians. The World Health Organization in 2005 ...
Salivary gland cancers are infrequent and pose a challenge owing to their histological diversity and varied clinical behavior, making the selection of optimal systemic treatments for advanced or recurrent stages difficult. This systematic review aims to assess overall survival outcomes and systemic treatment responses across four types of salivary cancers.
Introduction. Distant metastases (DMs) are the primary cause of treatment failure and death in patients with salivary gland carcinoma. 1, 2 The rates of distant failure range between 20% and 40%, with a higher rate for high-grade tumors. 3, 4 Because salivary gland carcinoma comprises a histologically heterogeneous group with variable biological behavior, the establishment of a uniform ...
Sclerosing mucoepidermoid carcinoma (SMEC) of the salivary glands is a rare variant of low-grade mucoepidermoid carcinoma with scanty cellular atypia characterized by marked fibrosis/sclerosis and a rich inflammatory infiltrate. Herein, we report 25 unpublished cases of SMEC, two of them with prominent eosinophilia (2/25; 8%) and three with abundant IgG4-positive plasma cells (3/25; 12%). In ...