
- Early Years
- Mental Health
- GCSE Maths Help
- GCSE English Help
- GCSE Business Help
- GCSE Psychology Help
- GCSE Physics Help
- GCSE Biology Help
- GCSE Chemistry Help

Calorimetry experiments

In this post
The amount of heat energy released by a chemical reaction can be measured using a method known as calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. The calorimetry experiments for these four types of reactions will now be described.
Combustion reactions are exothermic reactions. A fuel is a substance which releases heat energy when it is burned. Different fuels will release different amounts of heat energy. The heat energy released by the fuel is transferred to the surroundings.
To measure the heat energy released by a fuel, we burn a sample of the fuel with a known mass, below a glass or metal container full of water. The glass or metal container is known as a calorimeter.
The heat energy released from the combustion of the fuel is transferred to the water, causing the temperature of the water to increase. The method used to determine this temperature increase is described below:
- 50cm 3 of cold water is measured into a calorimeter.
- The initial temperature of the water is measured and recorded.
- A spirit burner containing the fuel is placed below the calorimeter and the wick is lit using a lit splint.
- The fuel is burned for a set amount of time (usually a couple of minutes).
- The highest temperature reached by the water is recorded.
During combustion reactions, the heat energy produced is transferred to the surroundings. This means that not all of the heat energy will be transferred directly into the water. Some will also be transferred to the container itself, as well as the air surrounding the container. The following steps should be taken to minimise this heat loss, and achieve measurements which are as accurate as possible:
- Use of shielding around the spirit burner to reduce heat loss to the air
- Use of a lid on the calorimeter to reduce heat loss through convection
- Use as small a distance as possible between the flame and container to reduce convection
When comparing the heat energy transferred by different fuels it is important that everything is kept the same in order to make it a fair test. The distance between the flame and calorimeter, the type of calorimeter used, the volume of water used and the amount of time the fuel is burned for should all be kept the same for each experiment completed.
Displacement reactions
A displacement reaction is one in which a more reactive metal replaces a less reactive metal in a compound. Displacement reactions are exothermic, so heat energy is released to the surroundings. If the metal added is in solid form and the metal compound is present as an aqueous solution, the amount of heat energy released can be calculated using the measured temperature increase of the solution of the metal compound.
The method used to determine the temperature increase is described below using the addition of zinc powder to copper sulphate solution as an example:
- Measure 50cm 3 of the copper sulphate solution into a polystyrene cup or glass beaker.
- Measure and record the initial temperature of the copper sulphate solution.
- Add a known mass of zinc powder to the copper sulphate solution and stir.
- Measure the temperature continually and record the highest temperature reached by the solution.
Dissolving occurs when a solid is added to a liquid and the bonds between the particles in the solid are broken, causing the solid particles to separate and spread out between the liquid particles.
One common type of dissolving experiment used is salts dissolving in water. Salts are ionic compounds. When they dissolve in water, the ions separate. Energy is required to overcome the forces of attraction between the ions in the solid form. This part of dissolving is endothermic.
When the ions have separated, they are able to spread out between the water particles. Forces of attraction are formed between the ions and the water molecules. This forming of the forces of attraction releases heat energy and is exothermic.
The dissolving of salts can be either an endothermic or exothermic change depending on how much energy is taken in to separate the ions in the solid compared to the energy released when the forces of attraction are formed between the ions and water molecules. The method for the calorimetry experiment is as follows:
- Place a polystyrene cup in a beaker.
- Measure and transfer 50cm 3 of water into the cup and measure the initial temperature.
- Add a known mass of the solid solute to the water in the cup and stir the solution to dissolve the solid.
- Once the solid has dissolved, measure and record the final temperature reached by the water.
Neutralisation
A neutralisation reaction is one in which an acid reacts with an alkali to produce water and a salt. For example, hydrochloric acid and sodium hydroxide solution react together, forming water and sodium chloride solution.
Neutralisation reactions are exothermic as the system releases heat energy to the surroundings. The system in a neutralisation reaction is the acid and alkali. The surroundings are the solution in which they are contained. When the acid and alkali react, they release heat energy into the surroundings, meaning that the temperature of the solution increases.
The temperature increase of the solution can be measured and used to calculate the amount of heat energy transferred during the reaction. The method for this experiment is describe below:
- Measure out 25cm 3 of the acid solution with a known concentration into a polystyrene cup. Place the cup into a glass beaker to prevent the cup being knocked over during the experiment.
- Measure and record the initial temperature of the acid.
- Measure out 25cm 3 of the alkali solution. This alkali solution should have the same concentration as the acid solution to make sure that the number of moles of acid and alkali used are equal.
- Add the 25cm 3 of alkali solution into the polystyrene cup and stir the solution.
- Measure and record the highest temperature reached by the solution.
Calculating the heat energy change
Once the temperature change has been measured from the calorimetry experiment, the amount of heat energy transferred can be calculated using the expression:
Energy transferred (Q) = mass of water (m) x specific heat capacity (c) x temperature change (△T)
= energy transferred (in J)
= mass of water or aqueous solution (in g)
= specific heat capacity of water (4.2J/g o C)
= temperature change (in o C)
The mass of water or aqueous solution used can be calculated using its volume and the density. The density of water is 1g/cm 3 . This means that 1cm 3 of water has a mass of 1g. 50cm 3 would therefore have a mass of 50g. Aqueous solutions are those made using water as a solvent. We therefore use the density of water to measure the mass of these solutions. For example, 50cm 3 of an aqueous solution would have a mass of 50g.
The specific heat capacity of a substance is the amount of energy needed to raise the temperature of 1g of the substance by 1 o C. The specific heat capacity of water is taken to be 4.2J/g. This means that it takes 4.2 Joules (J) of energy to raise the temperature of 1g of water by 1 o C.
The temperature change is the increase or decrease in temperature of the water or solution. This is calculated by subtracting the initial temperature from the final temperature. The heat energy change equation essentially tells us that the amount of heat energy transferred is equal to the mass of the water or solution that is heated, multiplied by the specific heat capacity of water (this is a constant and is equal to 4.2 joules for 1 gram of water) multiplied by the temperature change.
The heat energy change for any calorimetry experiment can be calculated using this equation. Worked example 1 on the next page shows how the equation can be applied and used in the example of a combustion reaction.
Ethanol in a spirit burner is burned below a calorimeter containing 50cm 3 of water. The initial temperature of the water was 21.5 o C and the highest temperature reached after heating was 82.5 o C. Calculate the heat energy change for the combustion of ethanol.
- Calculate the temperature change for the reaction.
The temperature of the water in the calorimeter before heating is 21.5. The temperature of this after heating is 82.5 To work out the temperature change we subtract the temperature before heating (21.5) from the temperature after heating (52.5).
82.5 – 21.5 = 61.0
- Substitute the values into the heat energy change equation:
Q = 50 x 4.2 x 31 = 12810J

Interested in a Chemistry GCSE?
We offer the Edexcel GCSE in Chemistry through our online campus.
Learn more about our Chemistry GCSE courses

Start your learning journey

Save your cart?

The Biology Corner
Biology Teaching Resources

Investigation: Heat Loss and Insulation in a Jar

This simple experiment can be used as a way to introduce the scientific method. Students design an experiment to test which materials are the best insulators by measuring heat loss.
The materials are simple, and the experiment doesn’t take very long. They will need two beakers per group, a thermometer, and hot water. Also, a variety of insulation materials, such as cotton, newspaper, styrofoam, cardboard.
You can substitute any number of materials. You can also brainstorm with students before class about things that make good insulators. Then, bring the ones students suggest for testing.
The Experiment
First, students design the experiment and discuss what type of data they will need to gather. They will need a beaker filled with hot water. I use a kettle to heat up water in my classroom.
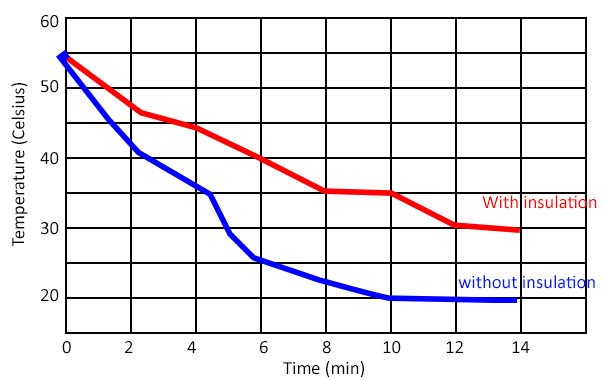
They will then use a thermometer to measure heat loss over 14 minutes. This is compared to another jar with a different type of insulation
Final Synthesis
Finally, students answer questions about their experiment. They identify the control, dependent, and independent variables. Then, they create a graph that shows how temperature changes over time. Students should observe that the beaker with insulation changes more slowly than the one without insulation. Instructors can compile all the group data so they can compare different materials and determine which one is the best insulator.
The graph shown below is a example of the type of graph that students will create based on their data. You can opt to run the experiment longer than 14 minutes which may have more dramatic results. The time is based on my class period which is about 50 minutes, leaving time for discussion and completing the worksheet.
Worksheet ends with a CER ( Claim, Evidence, Reasoning ). Students must answer the question about which materials are the best insulators.
There are two versions of this worksheet, a regular version with mostly open-ended questions, and a simpler version that has easier questions that are multiple choice. The simple version also has the graph set up (X and Y axes labeled). These versions are created for differentiation in classes that may have ELL students or students with special needs.
Shannan Muskopf

IMAGES
VIDEO
COMMENTS
What changes to the apparatus would make the experiment more accurate? The more accurate the thermometer used to take temperature measurements the better. A lid on the polystyrene cup also helps prevent heat loss.
You can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by recording and analyzing a temperature trace), but if it is negligible, you save that complication.
Barriers that conduct heat very poorly are good thermal insulators, whereas materials that conduct heat very well have a low insulating capability. In this activity, you will test which materials make good or bad thermal insulators with the help of a glass of hot water.
A simple experiment can be used to determine how much energy is lost to the calorimeter, the thermometer, and the surroundings. This energy loss, divided by the temperature change in the calorimeter that caused it, is often called the "calorimeter heat capacity" or "calorimeter constant" as illustrated by the following example:
Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions.
The addition of wall insulation prevents airflow, so heat loss (or gain) is decreased. On the other hand, the gap between the two panes of a double-paned window is about 1 cm, which largely prevents convection and takes advantage of air’s low conductivity reduce heat loss.
One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter).
Students design an experiment to test which materials are the best insulators by measuring heat loss. The materials are simple, and the experiment doesn’t take very long. They will need two beakers per group, a thermometer, and hot water.
Design and conduct an experiment to explore how insulation can be used to reduce heat transfer, e.g., make a container that holds ice cubes. Measure the temperature and amount of liquid after a specific period of time, and compare your results to those of classmates.
E5-1. The Task. In this experiment you will study the heat flow associated with a range of processes and examine the relationship between heat and temperature. Skills. By the end of the experiment you should be able to: use a temperature probe, understand how a calorimeter works. Other Outcomes.