

Understanding Percent Yield and Theoretical Yield
- The Albert Team
- Last Updated On: March 25, 2024

A fundamental concept that every budding chemist must grasp is calculating percent yield, a measure that bridges the theoretical world of chemistry with its practical applications. But what exactly is this value, and why is it so crucial in the realm of chemical reactions?
Percent yield is a key indicator used by chemists to determine the efficiency of a reaction. It compares the amount of product actually obtained from a reaction to the amount that theoretically could be produced. Understanding this concept is not just about crunching numbers; it’s about connecting the dots between the lab predictions and what transpires during a chemical reaction.
This post will guide you through the essentials of finding theoretical yield and calculating percent yield. Whether you’re conducting experiments in the lab or tackling problem sets in class, mastering these calculations will enhance your chemistry toolkit, enabling you to predict and analyze the outcomes of reactions with greater precision. So, let’s dive into the world of theoretical and percent yield, where the beauty of chemistry meets the rigor of mathematics!
What We Review
How to Find Theoretical Yield
Definition and importance.
Before we dive into the how-tos, let’s define theoretical yield. The definition is the amount of product that will theoretically be produced in a chemical reaction based on the limiting reactant and the stoichiometry of the reaction. It’s the maximum possible amount of product you can get under perfect conditions where everything goes exactly as planned.
But why is this theoretical amount important? In chemistry, predicting how much product a reaction can produce is essential. This prediction helps in planning and optimizing reactions, whether you’re synthesizing a new compound in a research lab or just performing a class experiment. Therefore, it’s all about efficiency and anticipation – knowing how much you can expect helps evaluate a reaction’s success and efficiency.
Breaking Down the Steps
With this in mind, let’s break down the steps:
- Balanced Chemical Equation : Everything starts with a balanced chemical equation. This equation tells you the ratio in which reactants combine and products form, which is crucial for the next steps.
- Identify the Limiting Reactant : The limiting reactant is the substance that will be used up first in the reaction, determining the maximum amount of product that can be formed. Compare the mole ratios of the reactants to figure out which one is the limiting reactant.
- Calculate Moles of Product : Using stoichiometry, convert the moles of the limiting reactant to moles of the desired product based on the coefficients in the balanced equation.
- Find Theoretical Yield : Finally, convert the moles of the product to grams (or any other unit, depending on the context) using its molar mass. This conversion gives you the theoretical yield of the product.
Let’s further illustrate this with a simple example. Imagine you’re reacting 2 moles of hydrogen ( \text{H}_2 ) with 1 mole of oxygen ( \text{O}_2 ) to produce water ( \text{H}_2\text{O} ). According to the balanced equation 2\text{H}_2 + \text{O}_2 \rightarrow 2\text{H}_2\text{O} , you can theoretically get 2 moles of water from 2 moles of hydrogen and 1 mole of oxygen. If hydrogen is your limiting reactant, then you can expect to produce 2 moles of water, which is your theoretical yield.
Finding Theoretical Yield: Practical Tips
Common challenges and solutions.
Finding the theoretical yield in a chemical reaction is a fundamental skill in chemistry, but it can come with its own set of challenges. For example, here are some common issues students might face and how to overcome them:
- Misinterpreting the Limiting Reactant : One common mistake is incorrectly identifying the limiting reactant. To avoid this, carefully compare the mole ratios of the reactants used to the ratios in the balanced equation. Remember, the limiting reactant is the one that would run out first, limiting the amount of product formed.

- Calculation Errors : When converting between moles and grams, double-check your calculations. A simple arithmetic mistake can throw off your entire result. Using a calculator and rechecking your steps can minimize these errors.
- Forgetting Units : Always include units in your calculations. Whether you’re working with moles, grams, or liters, keeping track of your units can prevent mix-ups and help ensure your calculations are correct.
Tools and Resources
In addition to understanding the common pitfalls, here are some tools and resources that can help you master finding theoretical yields:
- Stoichiometry Calculators : There are many online calculators available that can help you with stoichiometry problems. While it’s important to understand the process yourself, these tools can be useful for checking your work.

- Chemistry Textbooks and Guides : Don’t overlook the value of a good chemistry textbook or guide. These resources often provide step-by-step examples and can be great references when you’re stuck.
- Educational Videos and Tutorials : Visual learners might benefit from video tutorials available on educational platforms. The step-by-step process can provide a clearer understanding of how to find theoretical yield.
Overall, by being mindful of these challenges and utilizing available resources, you can enhance your ability to accurately find the theoretical amount of product in chemical reactions, a crucial skill in chemistry.
How to Calculate Percent Yield
Understanding the percent yield formula.
Percent yield is a crucial concept in chemistry, representing the efficiency of a reaction. The formula for this is:
- Actual Yield : The quantity of product actually produced in the reaction.
- Theoretical Yield : The amount of product predicted by stoichiometry.
This formula calculates the percentage of the predicted amount that was actually obtained in the experiment.
Step-by-Step Guide to Calculate Percent Yield
Follow these steps:
- Determine the Theoretical Yield : Use stoichiometry to find the theoretical yield, ensuring it is in the same units as the actual yield.
- Measure the Actual Yield : Obtain this from your experimental data.
- Apply the Percent Yield Formula : Insert your actual and theoretical yields into the percent yield formula to find the efficiency of your reaction.
Example Problem
If a reaction has a theoretical yield of 10.0 \text{ grams} and an actual yield of 8.0 \text{ grams} , the percent yield is calculated as:
This indicates that the reaction had an 80\% yield, meaning 80\% of the predicted product was successfully produced.
Practice Problem s
Test your knowledge with these practice problems on theoretical and percent yield. Solve the problems below before moving on to the answer section.
Practice Problem 1
Given the balanced equation 2 H_2 + O_2 \rightarrow 2 H_2O , calculate the theoretical yield of H_2O if you start with 5.0 moles of H_2 and an excess of O_2 .
Practice Problem 2
For the reaction P_4 + 6 Cl_2 \rightarrow 4 PCl_3 , if the theoretical yield of PCl_3 is 150.0 grams and the actual yield obtained from the experiment is 115.0 grams, calculate the percent yield.
Practice Problem 3
In the reaction N_2 + 3 H_2 \rightarrow 2 NH_3 , if you start with 10.0 moles of N_2 and 10.0 moles of H_2 , determine the limiting reactant and calculate the theoretical yield of NH_3 .
Practice Problem 4
Given the reaction C + 2 H_2 \rightarrow CH_4 , if you start with 12.0 grams of carbon and have an excess of hydrogen, first convert the mass of carbon to moles, determine the theoretical yield of methane in moles, and then calculate the percent yield if the actual yield is 22.0 grams of CH_4 .
Practice Problem Answers
Here are the solutions to the practice problems. Check your work and understand each step to improve your grasp of theoretical and percent yield calculations.
Practice Problem 1 Answer
For the equation 2 H_2 + O_2 \rightarrow 2 H_2O , with 5.0 moles of H_2 and an excess of O_2 , the theoretical yield of H_2O is calculated as follows:
Practice Problem 2 Answer
For the reaction P_4 + 6 Cl_2 \rightarrow 4 PCl_3 , with a theoretical yield of 150.0 grams and an actual yield of 115.0 grams, the percent yield is:
Practice Problem 3 Answer
In the reaction N_2 + 3 H_2 \rightarrow 2 NH_3 , starting with 10.0 moles of N_2 and 10.0 moles of H_2 , you must complete two stoichiometry problems, one for each reactant, to determine which would be the limiting reactant:
Since H_2 produces the least amount of NH_3 , it is the limiting reactant. This also tells you the theoretical yield, which will be 6.67 moles of NH_3 .
Practice Problem 4 Answer
For the reaction C + 2 H_2 \rightarrow CH_4 , converting 12.0 grams of carbon to moles:
The theoretical yield of CH_4 is 1.00 mole (since 1 mole of C produces 1 mole of CH_4 ). If the actual yield is 22.0 grams of CH_4 :
First, convert the amount obtained to moles:
Then, calculate the percent yield:
(Note: The percent can be over 100% due to measurement errors or impurities in the reactants.)
Congratulations on working through the fundamentals of theoretical and percent yield! These concepts are not just abstract numbers; they are essential tools that chemists use to measure the efficiency and success of their reactions. Understanding how to calculate theoretical helps you predict the maximum amount of product that can be produced in a reaction while determining percent yield gives you insight into the reaction’s efficiency in a real-world lab setting.
By mastering these calculations, you’re not just learning to crunch numbers—you’re gaining valuable skills that will help you in practical lab work and deepen your understanding of chemical processes. Remember, practice is key to becoming proficient in these concepts. The more you work through different problems and scenarios, the more intuitive these calculations will become.
So, keep challenging yourself with more complex reactions and different types of calculations. Your journey into the fascinating world of chemistry is just beginning, and these foundational skills will serve as stepping stones to more advanced topics and experiments. Keep exploring, stay curious, and enjoy the process of discovery in the wonderful world of chemistry!
Interested in a school license?
Popular posts.

AP® Score Calculators
Simulate how different MCQ and FRQ scores translate into AP® scores

AP® Review Guides
The ultimate review guides for AP® subjects to help you plan and structure your prep.

Core Subject Review Guides
Review the most important topics in Physics and Algebra 1 .

SAT® Score Calculator
See how scores on each section impacts your overall SAT® score

ACT® Score Calculator
See how scores on each section impacts your overall ACT® score

Grammar Review Hub
Comprehensive review of grammar skills

AP® Posters
Download updated posters summarizing the main topics and structure for each AP® exam.
1. Ensure you have a correctly balanced equation for the reaction performed. 2. Determine how many moles of each species were used in the reaction. 3. Determine which species is the limiting reagent, remembering to use the reaction stoichiometry. 4. From the weight of product obtained, determine how many moles of product this corresponds to. 5. Taking into account the stoichiometry, determine what % this is compared to what you could have obtained by 100 * [moles product obtained]/[ maximum moles product possible]
For example: 8.21g of cyclohexene was reacted with 17.5g of bromine in chloroform, giving 20g of trans -1,2-dibromocyclohexane.
Stoichiometry of this reaction is 1:1 Moles of each species involved: cyclohexene = 0.10 mol, Br 2 = 0.11 mol, dibromocyclohexane = 0.083 mol. Limiting reagent is therefore the cyclohexene. Theoretical yield of dibromocyclohexane is 0.10 mol., therefore, experimental yield = 0.083./0.10 = 83 %
Theoretical, experimental and percentage yield
The theoretical yield of a reaction is the amount of product you would get if you use up all of the limiting reagent, and if there is no loss, e.g. by degradation of reactant or product or by formation of byproducts. The experimental yield is the actual amount of product you obtain when performing a given experiment. The percentage yield is a calculation of how large a percentage of the theoretical yield you obtained in a given experiment. It is calculated by:
In most cases, when the yield of a reaction is mentioned without referring to either theoretical, experimental or percentage yield, it is implicitly the percentage yield that is meant.
- Understand the differences among actual yield, theoretical yield, and percent yield
- Calculate theoretical and percent yields.
DEFINITIONS:
4.4 Reaction Yields
Learning objectives.
By the end of this section, you will be able to:
- Explain the concepts of theoretical yield and limiting reactants/reagents.
- Derive the theoretical yield for a reaction under specified conditions.
- Calculate the percent yield for a reaction.
The relative amounts of reactants and products represented in a balanced chemical equation are often referred to as stoichiometric amounts . All the exercises of the preceding module involved stoichiometric amounts of reactants. For example, when calculating the amount of product generated from a given amount of reactant, it was assumed that any other reactants required were available in stoichiometric amounts (or greater). In this module, more realistic situations are considered, in which reactants are not present in stoichiometric amounts.
Limiting Reactant
Consider another food analogy, making grilled cheese sandwiches ( Figure 4.13 ):
Stoichiometric amounts of sandwich ingredients for this recipe are bread and cheese slices in a 2:1 ratio. Provided with 28 slices of bread and 11 slices of cheese, one may prepare 11 sandwiches per the provided recipe, using all the provided cheese and having six slices of bread left over. In this scenario, the number of sandwiches prepared has been limited by the number of cheese slices, and the bread slices have been provided in excess .
Consider this concept now with regard to a chemical process, the reaction of hydrogen with chlorine to yield hydrogen chloride:
The balanced equation shows the hydrogen and chlorine react in a 1:1 stoichiometric ratio. If these reactants are provided in any other amounts, one of the reactants will nearly always be entirely consumed, thus limiting the amount of product that may be generated. This substance is the limiting reactant , and the other substance is the excess reactant . Identifying the limiting and excess reactants for a given situation requires computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation. For example, imagine combining 3 moles of H 2 and 2 moles of Cl 2 . This represents a 3:2 (or 1.5:1) ratio of hydrogen to chlorine present for reaction, which is greater than the stoichiometric ratio of 1:1. Hydrogen, therefore, is present in excess, and chlorine is the limiting reactant. Reaction of all the provided chlorine (2 mol) will consume 2 mol of the 3 mol of hydrogen provided, leaving 1 mol of hydrogen unreacted.
An alternative approach to identifying the limiting reactant involves comparing the amount of product expected for the complete reaction of each reactant. Each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s stoichiometry. The reactant yielding the lesser amount of product is the limiting reactant. For the example in the previous paragraph, complete reaction of the hydrogen would yield
Complete reaction of the provided chlorine would produce
The chlorine will be completely consumed once 4 moles of HCl have been produced. Since enough hydrogen was provided to yield 6 moles of HCl, there will be unreacted hydrogen remaining once this reaction is complete. Chlorine, therefore, is the limiting reactant and hydrogen is the excess reactant ( Figure 4.14 ).
Link to Learning
View this interactive simulation illustrating the concepts of limiting and excess reactants.
Example 4.12
Identifying the limiting reactant.
Which is the limiting reactant when 2.00 g of Si and 1.50 g of N 2 react?
The provided Si:N 2 molar ratio is:
The stoichiometric Si:N 2 ratio is:
Comparing these ratios shows that Si is provided in a less-than-stoichiometric amount, and so is the limiting reactant.
Alternatively, compute the amount of product expected for complete reaction of each of the provided reactants. The 0.0712 moles of silicon would yield
while the 0.0535 moles of nitrogen would produce
Since silicon yields the lesser amount of product, it is the limiting reactant.
Check Your Learning
Percent yield.
The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical equation, is called the theoretical yield of the reaction. In practice, the amount of product obtained is called the actual yield , and it is often less than the theoretical yield for a number of reasons. Some reactions are inherently inefficient, being accompanied by side reactions that generate other products. Others are, by nature, incomplete (consider the partial reactions of weak acids and bases discussed earlier in this chapter). Some products are difficult to collect without some loss, and so less than perfect recovery will reduce the actual yield. The extent to which a reaction’s theoretical yield is achieved is commonly expressed as its percent yield :
Actual and theoretical yields may be expressed as masses or molar amounts (or any other appropriate property; e.g., volume, if the product is a gas). As long as both yields are expressed using the same units, these units will cancel when percent yield is calculated.
Example 4.13
Calculation of percent yield.
What is the percent yield?
Using this theoretical yield and the provided value for actual yield, the percent yield is calculated to be
How Sciences Interconnect
Green chemistry and atom economy.
The purposeful design of chemical products and processes that minimize the use of environmentally hazardous substances and the generation of waste is known as green chemistry . Green chemistry is a philosophical approach that is being applied to many areas of science and technology, and its practice is summarized by guidelines known as the “Twelve Principles of Green Chemistry” (see details at this website ). One of the 12 principles is aimed specifically at maximizing the efficiency of processes for synthesizing chemical products. The atom economy of a process is a measure of this efficiency, defined as the percentage by mass of the final product of a synthesis relative to the masses of all the reactants used:
Though the definition of atom economy at first glance appears very similar to that for percent yield, be aware that this property represents a difference in the theoretical efficiencies of different chemical processes. The percent yield of a given chemical process, on the other hand, evaluates the efficiency of a process by comparing the yield of product actually obtained to the maximum yield predicted by stoichiometry.
The synthesis of the common nonprescription pain medication, ibuprofen, nicely illustrates the success of a green chemistry approach ( Figure 4.15 ). First marketed in the early 1960s, ibuprofen was produced using a six-step synthesis that required 514 g of reactants to generate each mole (206 g) of ibuprofen, an atom economy of 40%. In the 1990s, an alternative process was developed by the BHC Company (now BASF Corporation) that requires only three steps and has an atom economy of ~80%, nearly twice that of the original process. The BHC process generates significantly less chemical waste; uses less-hazardous and recyclable materials; and provides significant cost-savings to the manufacturer (and, subsequently, the consumer). In recognition of the positive environmental impact of the BHC process, the company received the Environmental Protection Agency’s Greener Synthetic Pathways Award in 1997.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/4-4-reaction-yields
© Jun 3, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Actual Yield Definition in Chemistry

Actual yield is one of the types of yield in a chemical reaction, along with theoretical yield and percent yield. Here is the actual yield definition, how to find actual yield, and a look at why it’s always less than theoretical yield in an experiment.
Actual Yield Definition
Actual yield is the amount of product you experimentally obtain from a chemical reaction. In contrast, theoretical yield is the amount of product you obtain if all of the reactant converts to product. Actual yield is an empirical value that you measure in the lab, while theoretical yield is a calculated value.
How to Find Actual Yield
Usually, you find actual yield by weighing product using a scale:
- Weigh the container.
- Weigh the dry product in the container.
- Subtract the mass of the container from the total mass to get the mass of the product.
However, sometimes the product is measured indirectly in the unpurified reaction mixture. Measurements are taken via gas chromatography (GC), high-performance liquid chromatography (HPLC), nuclear resonance spectroscopy (NMR), or another analytical technique.
How to Calculate Actual Yield from Percent Yield
Another way of finding actual yield is from percent yield and theoretical yield.
percent yield = actual yield/theoretical yield x 100 actual yield = (percent yield x theoretical yield)/100
Isolated Yield
Many labs report isolated yield rather than actual yield. Isolated yield is the yield of product measured after it has been purified to a certain level (usually >95% spectroscopic purity). Because some product gets lost during purification, isolated yield tends to be lower than actual yield.
Reasons Why Actual Yield Is Less Than Theoretical Yield
Actual yield is lower than theoretical yield because most reactions aren’t 100% efficient and because it’s impossible to recover all of the product from a reaction. For example:
- Product remains on filter paper or passes through it.
- A tiny amount of product dissolves in a washing solvent, even if it’s insoluble in that solvent.
- Product that is a precipitate incompletely falls out of solution.
- Product evaporates.
Although less common, actual yield may be more than theoretical yield. Incompletely drying is the most common reason for this. Another reason is because an impurity is included in the product weight. Rarely, actual yield is higher than theoretical yield if another chemical reaction in the experiment also forms the same product.
Yield Inflation
In a 2010 Synlett article, Wernerova and Hudlický reported that the purification steps leading to isolated yield result in a loss of around 2% of product. Given the inherent loss, they concluded isolated yield rarely exceeds 94%. Yet, publications increasingly report higher and higher yields. This phenomenon is called yield inflation . There are multiple explanations for yield inflation.
- Improved techniques lead to higher yields.
- Small-scale reactions are more susceptible to slight measurement differences.
- Researchers artificially inflate yields to appear better in publication.
Assuming yield inflation is, in fact, a real phenomenon, the explanation is left for the reader to decide.
- McNaught, A. D.; Wilkinson, A., eds. (1997). Compendium of Chemical Terminology (the “Gold Book”) (2nd ed.). Oxford: Blackwell Scientific Publications. doi: 10.1351/goldbook ISBN 0-9678550-9-8.
- Petrucci, R.H., Harwood, W.S.; Herring, F.G. (2002) General Chemistry (8th ed.). Prentice Hall. ISBN 0130143294.
- Wernerova, Martina; Hudlicky, Tomas (November 2010). “On the Practical Limits of Determining Isolated Product Yields and Ratios of Stereoisomers: Reflections, Analysis, and Redemption”. Synlett . 2010 (18): 2701–2707. doi: 10.1055/s-0030-1259018
- Vogel, A. I.; Tatchell, A. R.; Furnis, B. S.; Hannaford, A. J.; Smith, P. W. G. (1996) Vogel’s Textbook of Practical Organic Chemistry (5th ed.). Pearson. ISBN 978-0582462366.
- Whitten, K.W., Gailey, K.D; Davis, R.E. (1992) General Chemistry (4th ed.). Saunders College Publishing. ISBN 0030723736.
Related Posts
Percent Yield Calculator
Table of contents
Our percent yield calculator will help you to understand how to calculate the percent yield , as well as teach you the percent yield formula and the percent yield definition .
Finding the yield is an integral part of any kind of synthetic lab work as the percent yield equation turns your experimental yields into a representation of how successfully you carried out your reaction .
Hopefully, after reading this page, you will have an answer to the questions "what is percent yield?" and "how to find percent yield?"
Percent yield definition
What is the percent yield? The percent yield definition is that it is a measure of the effectiveness of a synthetic procedure. Wordy, right? Simply put, percent yield tells you how well you carried out your reaction . If you were very careful with your reaction, made sure every molecule reacted and that nothing was lost when you poured the solution from one beaker to another, your percent yield would be 100 % 100\% 100% (don't worry if you don't get 100 % 100\% 100% , this is practically impossible). If you accidentally poured your reaction mixture down the drain and lost everything, then your percent yield would be 0 % 0\% 0% , and if you still had a lot of your solvent present with the product, your yield would be greater than 100 % 100\% 100% . Still confused about how to find the percent yield? Check the percent yield equation below!
A percent yield of 100 % 100\% 100% corresponds to the theoretical yield: discover this quantity with the theoretical yield calculator .
Percent yield formula
We can find percent yield can be found using the percent yield equation. It is expressed as a simple percentage calculated by using the experimental yield (we learned how to calculate it with our actual yield calculator ) of your product (i.e., how much of your product you made) and the theoretical mass of the product (i.e., the mass if not a single molecule was lost). The percent yield formula is:
- Y p Y_{\text{p}} Y p — The percent yield;
- m p , exp m_{\text{p},\text{exp}} m p , exp — The experimental mass of the product; and
- m p , th m_{\text{p},\text{th}} m p , th — The theoretical mass of the product.
The percent yield equation requires you to know two of the three variables, but it doesn't matter which two! Like any equation, it can be rearranged to find the unknown, but there's no need to worry about this when you can use our smart calculator; just enter the two known variables and find the third.
How to calculate percent yield
As you may have guessed from the percent yield equation above, if you want to know how to calculate the percent yield, you need two things, your experimental yield, and the theoretical yield. Let's assume you have both values; how to find the percent yield?
🙋 You may need to find the mole or concentration of your reactants to find the theoretical yield: use our tools, the mole calculator and the concentration calculator .
- First, make sure both weights have the same units (use our weight converter if you need some help 😉).
- Take your experimental yield and divide it by the theoretical yield.
- Multiply this value by 100 100 100 to find the percent yield.
There you go. Not too complicated, right? Or you could use our percent yield calculator to calculate it easily and quickly . A note about the values obtained; a value above 100 % 100\% 100% is possible but is due to solvent being present in the sample as well as your product. Dry your product thoroughly and re-weight it to get the true percent yield. Also, a value of 100% is impossible to achieve; there will always be some molecules that do not react or that are left on the side of the glassware. A value of 70 % 70\% 70% or higher is acceptable!
Examples of yield calculations
Time for some examples. Let's say you are doing a nucleophilic addition reaction, forming hydroxyacetonitrile from sodium cyanide and acetone.
Let's ignore the solvents underneath the arrow; we reacted 5 g 5\ \text{g} 5 g of acetone with 2 g 2\ \text{g} 2 g of cyanide, giving a theoretical yield of 6.54 g 6.54\ \text{g} 6.54 g of hydroxyacetonitrile. Now we know that if we carry out the experiment and get 5.58 g 5.58\ \text{g} 5.58 g of hydroxyactenitrile, what is the percent yield?
- We know our experimental yield is 5.58 g 5.58\ \text{g} 5.58 g , and our theoretical yield is 6.54 g 6.54\ \text{g} 6.54 g . Let's use the percent yield formula from above: Y p = m p , exp / m p , th ⋅ 100 Y_{\text{p}} =m_{\text{p},\text{exp}}/m_{\text{p},\text{th}}\cdot 100 Y p = m p , exp / m p , th ⋅ 100 and fill in the fields:
The percent yield is 85.3 % 85.3\% 85.3% . That was a pretty successful reaction! You should feel a bit more confident in calculating theoretical yield now. Let's try another example to bolster that confidence.

You react 8 g 8\ \text{g} 8 g of calcium carbonate with 9 g 9\ \text{g} 9 g of acetic acid, forming 4.35 g 4.35\ \text{g} 4.35 g of acetone. Nice! Now we do this reaction, but, unfortunately, we only get 1 g 1\ \text{g} 1 g of acetone. What is the percent yield?
- Our experimental yield is 1 g 1\ \text{g} 1 g , and our theoretical yield is 4.35 g 4.35\ \text{g} 4.35 g . Using the percent yield formula again gives us the following:
Now that's not great. Don't be upset, though. There's plenty of time left in the lab session, so you can try again. This time you try really, really hard not to lose any of your reaction mixture, and you end up with a yield of 5.31 g 5.31\ \text{g} 5.31 g . Well, that's much better than last time, so you carry out a percent yield calculation:
Oh no! The percent yield is over 100 % 100\% 100% , meaning there is still some solvent in our product. This means we need to dry our product further, so let's do that. After re-weighing our product (this time with no solvent), we find it weighs 4 g 4\ \text{g} 4 g . Let's calculate the percent yield:
Fantastic! Now you should have a grasp on the basics of percent yield calculation and, with it, have the knowledge you need to make the most out of our website. Happy calculating!
How do I calculate the actual yield given the percent yield?
To calculate the actual yield from the percent yield, you can use the following steps:
Use the formula for percent yield:
percent yield = (mass actual yield / mass theoretical yield) × 100% .
Rearrange to solve for the actual yield:
mass actual yield = (percent yield / 100%) × mass theoretical yield .
Substitute values and calculate the actual yield.
For instance, given a percent yield of 70% and a theoretical yield of 5 g, we can calculate the actual yield as follows:
mass actual yield = (70% / 100%)× 5 g . mass actual yield = 3.5 g
Can the percent yield be over 100%?
Yes. A percent yield value above 100% can occur due to solvent or other impurities present in the product or sample. Proper isolation and drying of the product are required to accurately measure the actual yield.

What's the difference between theoretical yield and actual yield?
Theoretical yield is the maximum amount of product that can be obtained from a chemical reaction, calculated based on the reaction's stoichiometry. Actual yield is the measured amount of product obtained from the reaction in the lab, and it's always expected to be lower than the theoretical yield due to factors like incomplete reactions, product loss, and impurities.
Is a percent yield of 100% possible?
No , in practice, it’s not possible to achieve a percent yield of 100%. Deviation below the theoretical yield is always expected due to factors such as impurities, incomplete reactions, and product loss. As a result, the actual yield will always be lower than the theoretical yield.
What's the actual yield, given a theoretical yield of 15 g?
Assuming a percent yield of 70%, the actual yield is 10.5 g . You can get this value by following these steps:
percent yield = (mass actual yield / mass theoretical yield) × 100%
mass actual yield = (percent yield / 100%) × mass theoretical yield
Substitute in the known values and calculate the actual yield:
mass actual yield = (70% / 100%) × 15 g mass actual yield = 10.5 g
Actual yield
Theoretical yield
Percent yield
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

- Percent Yield
Key Questions
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated, multiplied by 100%.
#"% yield" = ("actual yield")/("theoretical yield") * 100%#
So, let's say you want to do an experiment in the lab. You want to measure how much water is produced when 12.0 g of glucose ( #C_6H_12O_6# ) is burned with enough oxygen.
#C_6H_12O_6 + 6O_2 -> 6CO_2 + 6H_2O#
Since you have a #1:6# mole ratio between glucose and water, you can determine how much water you would get by
#12.0# #"g glucose" * ("1 mole glucose")/("180.0 g") * ("6 moles of water")/("1 mole glucose") * ("18.0 g")/("1 mole water") = 7.20g#
This represents your theoretical yield. If the percent yield is 100%, the actual yield will be equal to the theoretical yield. However, after you do the experiment you discover that only 6.50 g of water were produced.
Since less than what was calculated was actually produced, it means that the reaction's percent yield must be smaller than 100%. This is confirmed by
#"% yield" = ("6.50 g")/("7.20 g") * 100% = 90.3%#
You can backtrack from here and find out how much glucose reacted
#"65.0 g of water" * ("1 mole")/("18.0 g") * ("1 mole glucose")/("6 moles water") * ("180.0 g")/("1 mole glucose") = 10.8g#
So not all the glucose reacted, which means that oxygen was not sufficient for the reaction - it acted as a limiting reagent .
As a conclusion, percent yield problems always have one reactant act as a limiting reagent , thus causing a difference between what is calculated and what is actually obtained. A percent yield that exceeds 100% is never possible, under any circumstances, and means that errors were made in the calculations.

The actual yield is a product that is obtained by experimentation. The theoretical yield is obtained through stoichiometric calculation.
If the two yields are equal, you have 100 % yield.
Usually you obtain less than 100 %.
It is impossible to obtain a yield greater than 100%. If that happens, some experimental error must have occurred in the creation of the desired product, actual yield.

References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
Chapter 5. Stoichiometry and the Mole
Learning objectives.
1. Define and determine theoretical yields, actual yields, and percent yields.
In all the previous calculations we have performed involving balanced chemical equations, we made two assumptions: (1) the reaction goes exactly as written, and (2) the reaction proceeds completely. In reality, such things as side reactions occur that make some chemical reactions rather messy. For example, in the actual combustion of some carbon-containing compounds, such as methane, some CO is produced as well as CO 2 . However, we will continue to ignore side reactions, unless otherwise noted.
The second assumption, that the reaction proceeds completely, is more troublesome. Many chemical reactions do not proceed to completion as written, for a variety of reasons (some of which we will consider in Chapter 13 “Chemical Equilibrium” ). When we calculate an amount of product assuming that all the reactant reacts, we calculate the theoretical yield , an amount that is theoretically produced as calculated using the balanced chemical reaction.
In many cases, however, this is not what really happens. In many cases, less—sometimes much less—of a product is made during the course of a chemical reaction. The amount that is actually produced in a reaction is called the actual yield . By definition, the actual yield is less than or equal to the theoretical yield. If it is not, then an error has been made.
Both theoretical yields and actual yields are expressed in units of moles or grams. It is also common to see something called a percent yield. The percent yield is a comparison between the actual yield and the theoretical yield and is defined as

It does not matter whether the actual and theoretical yields are expressed in moles or grams, as long as they are expressed in the same units. However, the percent yield always has units of percent. Proper percent yields are between 0% and 100%—again, if percent yield is greater than 100%, an error has been made.
A worker reacts 30.5 g of Zn with nitric acid and evapourates the remaining water to obtain 65.2 g of Zn(NO 3 ) 2 . What are the theoretical yield, the actual yield, and the percent yield?
Zn(s) + 2 HNO 3 (aq) → Zn(NO 3 ) 2 (aq) + H 2 (g)
A mass-mass calculation can be performed to determine the theoretical yield. We need the molar masses of Zn (65.39 g/mol) and Zn(NO 3 ) 2 (189.41 g/mol). In three steps, the mass-mass calculation is

Thus, the theoretical yield is 88.3 g of Zn(NO 3 ) 2 . The actual yield is the amount that was actually made, which was 65.2 g of Zn(NO 3 ) 2 . To calculate the percent yield, we take the actual yield and divide it by the theoretical yield and multiply by 100:

The worker achieved almost three-fourths of the possible yield.
Test Yourself
A synthesis produced 2.05 g of NH 3 from 16.5 g of N 2 . What is the theoretical yield and the percent yield?
N 2 (g) + 3 H 2 (g) → 2 NH 3 (g)
theoretical yield = 20.1 g; percent yield = 10.2%
Chemistry Is Everywhere: Actual Yields in Drug Synthesis and Purification
Many drugs are the product of several steps of chemical synthesis. Each step typically occurs with less than 100% yield, so the overall percent yield might be very small. The general rule is that the overall percent yield is the product of the percent yields of the individual synthesis steps. For a drug synthesis that has many steps, the overall percent yield can be very tiny, which is one factor in the huge cost of some drugs. For example, if a 10-step synthesis has a percent yield of 90% for each step, the overall yield for the entire synthesis is only 35%. Many scientists work every day trying to improve percent yields of the steps in the synthesis to decrease costs, improve profits, and minimize waste.
Even purifications of complex molecules into drug-quality purity are subject to percent yields. Consider the purification of impure albuterol. Albuterol (C 13 H 21 NO 2 ; accompanying figure) is an inhaled drug used to treat asthma, bronchitis, and other obstructive pulmonary diseases. It is synthesized from norepinephrine, a naturally occurring hormone and neurotransmitter. Its initial synthesis makes very impure albuterol that is purified in five chemical steps. The details of the steps do not concern us; only the percent yields do:
That is, only about one-fourteenth of the original material was turned into the purified drug. This gives you one reason why some drugs are so expensive; a lot of material is lost in making a high-purity pharmaceutical.
Key Takeaways
- Theoretical yield is what you calculate the yield will be using the balanced chemical reaction.
- Actual yield is what you actually get in a chemical reaction.
- Percent yield is a comparison of the actual yield with the theoretical yield.
What is the difference between the theoretical yield and the actual yield?
What is the difference between the actual yield and the percent yield?
A worker isolates 2.675 g of SiF 4 after reacting 2.339 g of SiO 2 with HF. What are the theoretical yield and the actual yield?
SiO 2 (s) + 4 HF(g) → SiF 4 (g) + 2 H 2 O(ℓ)
A worker synthesizes aspirin, C 9 H 8 O 4 , according to this chemical equation. If 12.66 g of C 7 H 6 O 3 are reacted and 12.03 g of aspirin are isolated, what are the theoretical yield and the actual yield?
C 7 H 6 O 3 + C 4 H 6 O 3 → C 9 H 8 O 4 + HC 2 H 3 O 2
A chemist decomposes 1.006 g of NaHCO 3 and obtains 0.0334 g of Na 2 CO 3 . What are the theoretical yield and the actual yield?
2 NaHCO 3 (s) → Na 2 CO 3 (s) + H 2 O(ℓ) + CO 2 (g)
A chemist combusts a 3.009 g sample of C 5 H 12 and obtains 3.774 g of H 2 O. What are the theoretical yield and the actual yield?
C 5 H 12 (ℓ) + 8 O 2 (g) → 5 CO 2 + 6 H 2 O(ℓ)
What is the percent yield in Exercise 3?
What is the percent yield in Exercise 4?
What is the percent yield in Exercise 5?
What is the percent yield in Exercise 6?
Theoretical yield is what you expect stoichiometrically from a chemical reaction; actual yield is what you actually get from a chemical reaction.
theoretical yield = 4.052 g; actual yield = 2.675 g
theoretical yield = 0.635 g; actual yield = 0.0334 g
- Introductory Chemistry- 1st Canadian Edition . Authored by : Jessie A. Key and David W. Ball. Provided by : BCCampus. Located at : https://opentextbc.ca/introductorychemistry/ . License : CC BY-NC-SA: Attribution-NonCommercial-ShareAlike . License Terms : Download this book for free at http://open.bccampus.ca

Privacy Policy
Precision drip Irrigation System and Foliar Application of Biostimulant and Fertilizers Containing Micronutrients Optimize Photochemical Efficiency and Grain Yield of Maize ( Zea mays L)
- Original Paper
- Open access
- Published: 30 October 2024
Cite this article
You have full access to this open access article

- Akasairi Ocwa ORCID: orcid.org/0000-0003-4787-9270 1 , 2 ,
- Csaba Bojtor ORCID: orcid.org/0000-0001-5870-9977 1 ,
- Árpád Illés ORCID: orcid.org/0000-0001-6548-2926 1 ,
- Brian Ssemugenze ORCID: orcid.org/0009-0007-9263-018X 1 , 2 ,
- Ibtissem Balaout 1 , 2 ,
- Tamás Rátonyi 1 ,
- Adrienn Széles 1 &
- Endre Harsányi ORCID: orcid.org/0000-0002-8931-4297 1 , 3
Asymmetric drought propagation and depletion of soil nutrients threaten cereal crop productivity worldwide, calling for the application of validated agronomic practices to curtail their effect on crop production. This study evaluated the effect of precision drip irrigation, biostimulant, and micronutrients application on photochemical efficiency and yield of maize.An experiment laid in a randomized complete block design with irrigation and water stress was established in 2022 and 2023 growing seasons at the experimental area of the University of Debrecen. Other treatments included T1 (non-microbial biostimulant from plant origin), T2 (zinc based chemical fertilizer), T3 (boron and molybdenum based chemical fertilizer), and T4 (control). Data was collected on steady-state fluorescence (F’), maximal fluorescence (F m ’), quantum photosynthetic yield or efficiency of photosystem II (ΦPSII or Y(II)), electron transport rate (ETR), and grain yield and yield components. Precision drip irrigation significantly optimized ΦPSII, ETR, cob weight, number of seeds per cob, weight of 1000 seeds and grain yield. The biostimulant and micronutrients optimized F m ’, ΦPSII, and ETR at VT and R2 growth stages. Regardless of the water management regime, T1, T2 and T3 seasonally optimized grain yield. Between water management regimes, biostimulant had the highest yield optimization effect under precision drip irrigation in the season with elevated water stress.Optimum photochemical efficiency and grain yield is achievable through precision drip irrigation, biostimulant, and micronutrient application. However, further research involving 2–3 application times at critical stages of maize under precision drip irrigation and/or combined application of these treatments at season specific precision drip irrigation is required.
Avoid common mistakes on your manuscript.
1 Introduction
Maize ( Zea mays L) is among the most important cereal crops, after rice ( Oryza sativa L) and wheat ( Triticum aestivum L ) worldwide due to its economic and nutritional benefits (Prasanna et al. 2021 ; Ssemugenze et al. 2024 ). However, abiotic stresses such as water stress (extreme drought) and heat stress (Simkó et al. 2020 ) negatively impact its production and productivity. Moreover, these climate extremes are expected to increase globally (Zampieri et al. 2019 ; Simkó et al. 2020 ). A simulation by Zampieri et al. ( 2019 ) revealed that temperature rise by 2 °C in the late 2030s will affect maize production in both minor and major production areas like never experienced before. There exists a negative nexus between morphological, physiological, and biochemical changes in maize with drought and/or heat stress (Trivedi et al. 2018 ; Rehaman et al. 2023 ). In particular, drought stress significantly affects the nutritional status of maize (Ge et al. 2010 ; Klofac et al. 2023 ); hence, balanced supply of adequate mineral nutrients is pivotal (Kovács et al. 2009 ; Waraich et al. 2011 ; Klofac et al. 2023 ). Research has shown that although maize as a C 4 plant efficiently utilizes moisture under limited supply, its productivity is still reduced by drought, depending on the crop stage it onsets (Killi et al. 2017 ; Hussain et al. 2020 ). Kumar et al. ( 2024 ) noted that supply of moisture at stress-sensitive growth stage of crops enhances productivity. This calls for the application of precision water delivery technologies (Chen et al. 2023 ). Conversely, different fertilizer formulations are produced and applied in crop production to alleviate nutrient stress and increase productivity (Bojtor et al. 2021 ; Ocwa et al. 2023 ), though often micronutrients are neglected. For example, zinc is a major essential micronutrient, which in low supply limits crop growth (Ghani et al. 2022 ), and yield (Ma et al. 2017 ).
Supplementation of maize with nutrients such as zinc improves yield (Zhang et al. 2020 ), and zinc deficiency causes over 40% crop yield reduction (Noulas et al. 2018 ; Idrees et al. 2024 ). Besides, zinc plays a fundamental role in crop resistance against drought stress by regulating various physiological processes (Klofac et al. 2023 ). Though abundant in most soils, its uptake by plants is limited by several physicochemical soil conditions (Alloway, 2009 ) making it the most deficient element especially in certain soil types (Sadeghzadeh, 2013 ). On the other hand, despite the fact that boron requirement by maize is relatively low, it is one of the yield limiting factors in some geographic regions (Hayat et al. 2023 ). Conversely, besides chemical fertilizers, biostimulants are used to boost crop productivity (Abbott et al. 2018 ; Sible et al. 2021 ; Ocwa et al. 2024 ). Despite the use of these inputs, earlier, it was underscored that photosynthesis interference by drought and/or heat (Bresson et al. 2015 ; Dogru, 2021 ; Nematpour and Eshghizadeh, 2023 ), and nutrient stress (Rácz et al. 2021 ; Ocwa et al. 2024 ) decreases yield (Bresson et al. 2015 ). This necessitates undertaking critical assessments during the crop growth cycle to unravel the effects of applied agronomic interventions.
Chlorophyll fluorescence measurements are pivotal in monitoring the response of plants to stress (Sinsawat et al. 2004 ; Dogru, 2021 ) and amelioration capacity of agronomic interventions such as fertilization and irrigation. Among the vital parameters include minimal fluorescence (Fo), maximum fluorescence (Fm), variable fluorescence (Fv), and photochemical efficiency of photosystem II. According to Chen et al. ( 2019 ), chlorophyll florescence, photochemical reactions and other pathways utilize light energy in the photosystem II (PSII). As such, fluorescence yield indicates the fraction of absorbed light energy re-emitted as fluorescence while the photochemical yield of PSII is an indication of photochemical light use efficiency since it’s the fraction of absorbed photons used for photochemical reactions (Chen et al. 2019 ). According to Janušauskaite and Feiziene ( 2011 ), the efficiency of cereal crop photosystems depends on weather conditions. Rehaman et al. ( 2023 ) revealed that maximum quantum efficiency of PSII, ETR and intrinsic PSII activity significantly reduced due to drought stress by 11.3, 14.5, and 10.8%, respectively. Likewise, a report by Cendrero-Mateo et al. ( 2016 ) revealed a significant decrease in photochemical yield and steady-state fluorescence due to drought. Similarly, Arikan et al. ( 2022 ) reported a decrease in Fv/Fo (activity of the water-splitting complex on the donor site of the PSII and Fv/Fm by 48.6 and 14.1%, respectively under water stress compared to the control. Killi et al. ( 2020 ) noted that maize varieties tolerant to drought retained PSII function at the temperatures of 25 and 35°C treatments at limited water supply. Besides drought stress, fluorescence parameters can vary depending on plant nutrition (Tubuxin et al. 2015 ; Chen et al. 2016 ). In fact, nutrients supplied exogenously by biostimulants and/or chemical fertilizers affect chlorophyll fluorescence parameters differently, depending on several factors. Li et al. ( 2012 ) noted that the electron donor and acceptor performance in the reaction center of PSII was significantly increased by nitrogen application. Toth et al. ( 2022 ) indicated that the application of zinc and amino acids had no significant effect on Fo, reduced Fm while zinc alone reduced Fo. Some reports revealed higher Fm, Fv and Fv/Fm value in zinc application except Fo that was not affected (Wang and Jin, 2005 ; Guo and Tan 2015 ). Besides, Klofac et al. ( 2023 ) reported that quantum yield significantly increased after foliar application of zinc oxide micro (ZnO). Moreover, there exist a nexus between photochemical parameters and grain yield. Earlier, a comprehensive review by Guo and Tan ( 2015 ) revealed that although chlorophyll fluorescence has successfully been applied in plant research, inconsistencies in some results are still reported. Among the causes, include the type of equipment, light conditions and other abiotic stresses. Specifically, plant leaves exposed to high actinic light levels for several hours resist closure of PSII reaction centers even though high saturation light pulse is applied, hence affecting reliability of ΦPSII and ETR measurements (Loriaux et al. 2013 ). It is proposed that the solution is to apply the method called F m ’ correction that uses multiple phased saturation flash available in Opti-Sciences such as Y(II) meter, OS1p, OS5p and OS5p+ (Loriaux et al. 2013 ); hence this study utilized OS5p + equipment. With this in mind, currently in Hungary, agro-technical innovations have been developed to ameliorate drought, heat and nutrient stresses in attempt to optimize maize production amidst unprecedented climate change. Hence, this study evaluated the effect of precision drip irrigation, biostimulant and chemical fertilizers containing micronutrients on chlorophyll fluorescence, photochemical efficiency and grain yield of maize.
2 Materials and Methods
2.1 experimental site.
The experiments were carried out at the University of Debrecen, Böszörményi street experimental area (47°83, 030”N, 21°82, 060”E, 111 m asl). The season 2022 had elevated temperature and low precipitation between May to August, which were the active months of maize growth. In fact, the temperature was elevated by close to 1 o C in May, July, and August and, 2.4 o C in June while precipitation reduced by close to -1 mm in May and July, and − 2 mm in June and August in 2022 compared to 2023 season. Generally, the precipitation in each of the months May to August was < 0.8 mm in 2022 season and > 1 mm in 2023 season (Fig. 1 ). The soil analysis and nutrient adequacy and/or deficiency interpretation was based on the Hungarian New Fertilization Guidelines (Antal et al. 1987 ). The soil type was leached chernozem. The soil in the experimental site had 5.3 mg kg –1 available nitrogen, 320.9 mg kg –1 available phosphorus and 256.2 mg kg –1 available potassium. The concentration of other essential elements and the pH of the soil are presented in Table 1 .

Temperature ( a ) and the amount of precipitation received ( b ) during experimental period
2.2 Experimental Design and Treatments
The experiment was laid in a randomized complete block design (RCBD) with four replications. Experimental plots measured 10 m 2 each (32 plots in total), and water treatment had two levels: irrigated (IR) and water stress (WS). Precision drip irrigation system was installed on 27 th May 2022 and 12 th June 2023 prior to onset of drought and removed when maize attained physiological maturity. Based on the meteorological data, the installation was delayed by 15 days in 2023 because of adequate moisture in the soil. All irrigated plots were supplied with the same amount of water while water stress plots were not irrigated even when maize plants were at wilting point during drought (summer). Each row of maize had one drip irrigation line/strip. Soil moisture content was measured using a Campbell wet sensor at different depths (-0.1 m and − 0.3 m). Irrigation was started at the minimum value of the maximum field water capacity value. The intensity of the irrigation was 3 l/m 2 / hour. If the probability of rain reached 80%, no irrigation was started. Irrigation was done between 9pm to 5am to reduce evapotranspiration. Total amount of water applied during the whole experimental period was 413.6 mm and 358 mm in 2022 and 2023 growing seasons, respectively. The drip irrigation system was precision-managed by remote control via a mobile phone application (Hydrawise application (Hunter)), where the amount of water applied, and intensity was continuously monitored. Biostimulant and chemical fertilizer treatments were designated as T1, T2, T3, and T4 (control). Detailed composition of the treatments and their application rates as recommended by the manufacturer are provided in Table 2 . Treatments were applied once using a motorized pump at V8 stage when maize plants had attained large leaf surface area to absorb nutrients.
2.3 Seedbed Preparation, Sowing, and General Agronomic Practices
The seedbed was prepared using a KongsklideVibro Master SGC/SQ25 mounted seedbed cultivator (Kongskilde Agriculture, Albertslund, Denmark). Prior to planting, fertilizer was applied in the soil at the rate of 101.3 kgha −1 N, 26.3 kgha −1 CaO, 18.8 kgha −1 MgO on 11 th April 2022 (2022 season) and 90.0 kgha −1 N, 23.0 kg ha −1 CaO, and 16.0 kg ha −1 MgO on 31 st March 2023 (2023 season).The seeds of maize hybrid FAO 420 were sown on 2 nd May 2022 and 28 th April 2023 using a Gaspardo MTR4 pneumatic precision seed drill (Maschio Gaspardo S.p.A., Campodarsego, Italy) with a spacing of 76.2 × 18.2 cm at a seed rate of one seed per hole, giving total plant population of 72,100 per hectare. FAO 420 hybrid was used because it is widely cultivated on a large scale in Hungary. In both seasons weed management was done as recommended.
2.4 Chlorophyll Fluorescence Measurements
Data was collected on chlorophyll fluorescence parameters such as F’, F m ’ , ΦPSII and ETR. Chlorophyll fluorescence parameters were measured using Pulse Modulated Chlorophyll Fluorometer (PerkinElmer Inc., Waltham, MA, USA) at the V12 (twelve-leaf stage) VT (tasseling stage) and R2 (kernel blister stage) growth stages. In each plot, four plants were randomly selected (Yin et al. 2011 ), and measurements taken in the new fully expanded leaf of each plant at vegetative stage and leaf opposite the ear at the reproductive stage, respectively (Simkó et al. 2020 ). For saturation pulse intensity, prior to measurement, the selected leaves from each plant were dark adapted for 30 minutes (Hu et al. 2023 ). Measurements were taken for: F ’ which is the steady-state fluorescence signal under actinic light prior to saturation pulse, F m ’ which is maximal fluorescence under actinic light at steady state photosynthesis when all the reaction centers are closed. The fluorescence difference between F m ’ and F ’ (F m ’ - F ’ ) constituted Fq ’ (Baker, 2008 ; Janka et al. 2015 ) . The ΦPSII which is the quantum photosynthetic yield (efficiency) of PSII was calculated as (F m ' - F ' )/F m ' (Baker, 2008 ; Janka et al. 2015 ; Hazrati et al. 2016 ). The ETR, which is an estimate of the number of electrons transported through photosystem II under steady-state photosynthetic conditions, was calculated using the formula indicated in the manufacturer’s protocol as well as in published literature (Flexas et al. 2002 ; Hu et al. 2023 ). The equation used was:
Where 0.84 is average leaf absorptance value of PSII, and 0.5 means two photons used to excite one electron (Flexas et al. 2002 ), and PAR is photosynthetically active radiation. All measurements were taken at the temperature of 25 o C (Yin et al. 2011 ) between 10 a.m. and 1 p.m.
2.5 Yield and Yield Components
Harvesting was done after the appearance of the black layer in the grains. Ten ears were randomly collected from 10 plants from each replication and processed using HALDRUP LT-35 laboratory thresher (HALDRUP GmbH, Ilshofen, Germany). Cob weight (g) was determined using electronic weighing balance. The number of seeds and 1000-seed weight (g) were determined using VSC-201 Vibrating Seed Counter (PLC Tuning Ltd - Hungary) and grain yield (GY) in t ha –1 calculated at moisture content of 14.5%.
2.6 Statistical Data Analyses
The normality and homogeneity of variance of data was tested using Shapiro Wilk and Levene’s tests, respectively. Data was analyzed using OriginPro Graphing and Analysis Software (version 2024) and R software (version 4.3.2). The two-way ANOVA was implemented and the differences between treatment means were compared using Tukey test at 5% probability level. The performance of the water management regimes was compared using the t-test. The Pearson correlation was used to assess the nexus between chlorophyll fluorescence, photochemical and grain yield. Figures were prepared using OriginPro Graphing and Analysis Software and R software.
3.1 Response of Chlorophyll Fluorescence and Photochemical Yield of Maize to Water Management, and Foliar Biostimulant and Micronutrients Application
The chlorophyll fluorescence and photosynthetic efficiency significantly ( p < 0.05) differed between precision drip irrigation and water stress treatments in both seasons. At the V12 growth stage in 2022 growing season, significantly lower F m ’, Fq ’ , ΦPSII, and ETR were recorded under precision drip irrigation compared to water stress. Contrary, at VT growth stage, precision drip irrigation optimized F m ’, Fq ’ , ΦPSII, and ETR by 23.4, 33.5, 10.4, and 10.4%, respectively. At R2 stage, ΦPSII and ETR were significantly improved by precision drip irrigation by 6.7 and 6.9%, respectively. In 2023 growing season, precision drip irrigation significantly improved chlorophyll fluorescence and photochemical yield at VT and R2 growth stages, respectively. At VT growth stage, F m ’, Fq ’ , ΦPSII and ETR were improved by 11.5, 15.2, 2.9 and 3.8%, respectively. At R2 growth stage, precision drip irrigation significantly improved both ΦPSII and ETR by 3.9%. Progressively, F’ reduced at the VT stage under precision drip irrigation and water stress in both seasons. Meanwhile, as water stress reduced F m ’, Fq ’ , ΦPSII, and ETR during the VT growth stage, precision drip irrigation optimized these parameters. The progress of chlorophyll and photochemical parameters is shown in Fig. 2 .
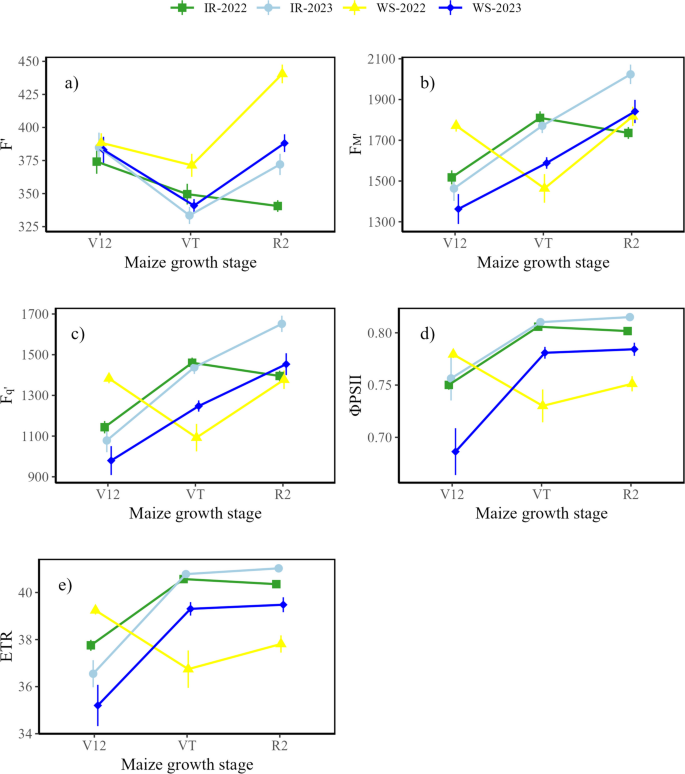
Dynamics of chlorophyll fluorescence and photochemical yield under precision irrigation and water stress at different maize growth stages in 2022 and 2023 growing seasons. a ) F ’ (steady-state fluorescence signal under actinic light prior to saturation pulse), b ) FM’ (maximal fluorescence under actinic light at steady state photosynthesis when all the reaction centers are closed), c ) Fq ’ (fluorescence difference between FM’ and F ’ ), d ) ΦPSII (quantum photosynthetic yield (efficiency) of photosystem II, e ) ETR (electron transport rate). Vertical bars indicate the standard error
Significant changes in chlorophyll fluorescence parameters (F’, F m ’ and Fq ’ ) in response to biostimulant and micronutrients application depended on the growth stage. In both seasons, F’ did not significantly ( p > 0.05) differ including the interactive effects. As such, in both seasons, the F’ value of the treatments ranged from 335–435 and 320–410, respectively, under both precision drip irrigation and water stress in all the stages. Conversely, biostimulant and micronutrients application significantly ( p < 0.05) affected F m ’ at the R2 growth stage under water stress in 2022 season, and at VT and R2 under both water management regimes in 2023 season. At the R2 growth stage in 2022 season under precision drip irrigation, all the treatments had F m ’ values ranging from 1659–1733 while under water stress, only T1 and T2 had significantly higher values of 1870, 1833 compared to 1795 and 1773 in T3 and the control, respectively. In 2023 season at the VT growth stage, the F m ’ was 1887, 1730 and 1831 in T1, T2 and T3, respectively compared to 1636 in the control under precision drip irrigation while under the water stress, only T1 had significantly higher F m ’ value of 1670 compared to 1512, 1569 and 1603 in T2, T3 and the control, respectively. The same trend was followed in R2 growth stage. In terms of the Fq ’ , only T1 had a significant ( p < 0.05) effect at R2 growth stage in 2022 season. The Fq ’ was 1413 in T1 compared to 1397, 1343 and 1355 in T2, T3 and the control, respectively under water stress while under precision drip irrigation, all treatments had similar performance. In the 2023 season, T1 had significantly better performance at VT and R2 stages. At the VT growth stage, the Fq ’ was 1544, 1403, and 1491 in T1, T2, and T3 compared to 1313 in the control, respectively, under precision drip irrigation while under water stress, Fq ’ was 1327, 1177 and 1230 in T1, T2 and T3 compared to 1257 in the control, respectively. Similarly, at the R2 growth stage, T1 still had higher Fq ’ of 1721 compared to 1539, 1681 and 1665 in T2, T3 and the control under precision drip irrigation while under water stress, T1 maintained higher Fq ’ of 1530 compared to 1269, 1581 and 1434 in T2, T3 and the control.
Although the individual effects of the biostimulant and micronutrients within each water regime were not significant, the interactive effects between biostimulant and micronutrients × water management on ΦPSII and ETR were significant at VT and R2 growth stages in both seasons. At the VT growth stage in 2022 season, T1 under drip precision irrigation had significantly higher (0.88) ΦPSII as compared to 0.75 and 0.72 in T2 and T3 under water stress, respectively. Similarly, T3 under precision irrigation had higher (0.85) ΦPSII compared to 0.72 in T3 under water stress. At the R2 growth stage, all the treatments still had higher ΦPSII under precision irrigation compared to their performance under water stress. Equally, in 2023 season, T1, T2 and T3 had higher ΦPSII under precision drip irrigation compared to their efficacy under water stress. However, more peculiar was that T2 under water stress had higher reduction of ΦPSII compared to all other treatments under precision drip irrigation both at the VT and R2 growth stages (Fig. 3 ). On the other hand, like the ΦPSII, the significant interactive effects revealed higher ETR of treatments T1, T1 and T3 under precision drip irrigation compared to water stress. In fact, at the VT and R2 growth stages in both seasons, only T1 maintained a higher ETR. It is also worthwhile to note that in both seasons, no significant effects on ETR were recorded at the V12 growth stage (Fig. 4 ).
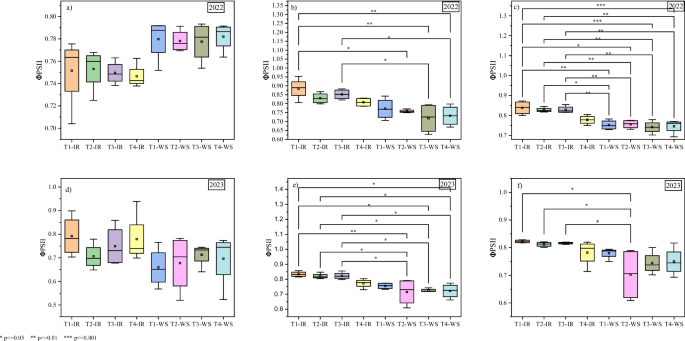
Quantum photosynthetic yield (efficiency) of photosystem II (ΦPSII), under biostimulant and micronutrient treatments for precision drip irrigation and water stress conditions at different stages of maize growth. ( a ) V12 growth stage in 2022 season, ( b ) VT growth stage in 2022 season, ( c ) R2 growth stage in 2022 season, ( d ) V12 growth stage in 2023 season, ( e ) VT growth stage in 2023 season, ( f ) R2 growth stage in 2023 season. IR – Precision drip irrigation, WS – Water stress, T1 – biostimulant, T2 and T3 – chemical fertilizers containing micronutrients (details in the methodology), T4 – control. *, **, and *** means significant differences by Tukey test at p < 0.05, p < 0.01, and p < 0.001, respectively (treatments not compared were not significantly different)
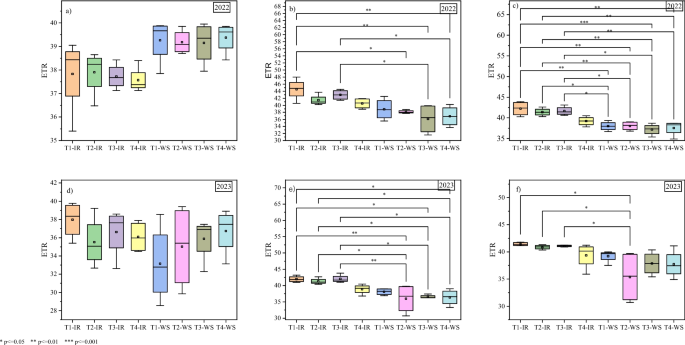
Electron transport rate (ETR) under biostimulant and micronutrient treatments for precision drip irrigation and water stress conditions at different stages of maize growth. ( a ) V12 growth stage in 2022 season, ( b ) VT growth stage in 2022 season, ( c ) R2 growth stage in 2022 season, ( d ) V12 growth stage in 2023 season, ( e ) VT growth stage in 2023 season, ( f ) R2 growth stage in 2023 season. IR – Precision drip irrigation, WS – Water stress, T1 – biostimulant, T2 and T3 – chemical fertilizers containing micronutrients (details in the methodology), T4 – control. *, **, and *** means significant differences by Tukey test at p < 0.05, p < 0.01, and p < 0.001, respectively (treatments not compared were not significantly different)
3.2 Effect of Water Management, Foliar Biostimulant and Micronutrients Application on Yield and Yield Components of Maize
3.2.1 number of seeds per cob, weight of 1000 seeds and cob weight.
In both seasons, water management significantly ( p < 0.05) improved the number of seeds per cob, with precision drip irrigation having 538 and 506 seeds per cob compared to 164 and 468 seeds per cob under water stress in 2022 and 2023 seasons, respectively. The individual effects of treatments T1, T2 and T3 under each water regime and their interactions with water management did not significantly differ in the two seasons. In 2022 season under precision drip irrigation, the seed number per cob was 573, 529 and 571 in T1, T2 and T3 compared to 478 in the control. Similarly, under water stress conditions, the seed number per cob was 142, 194 and 177 in T1, T2 and T3 compared to 144 in the control. This similar trend was recorded in the 2023 season. In terms of weight of 1000 seeds, precision drip irrigation significantly improved 1000 seed weight both in 2022 and 2023 seasons. Precisely, the weight of 1000 seeds under precision drip irrigation was 363.3 and 534.7 g compared to 268.3 and 505.2 g under water stress. Meanwhile, the treatment and interaction effects were only significant for 2023 season. In 2022 season, all treatments (not significant) had 1000 seed weight between 312.5 and 318.6 g while in 2023, treatments T1, T2 and T3 had 1000 seed weight of 537.3, 531.6 and 525.2 g compared to 485.4 g in the control. Interactively, T2 had the highest (569.9 g) 1000 seed weight under precision drip irrigation (Fig. 5 ). Looking at cob weight, in both 2022 and 2023 seasons, precision drip irrigation significantly improved cob weight with weight of 222.19 and 304.6 g compared to 57.3 and 261.9 g under water stress. On the other hand, although the individual effects of the biostimulant and micronutrients within each water regime did not significantly differ, specific comparison of their performance between the two water management regimes revealed a higher cob weight under precision drip irrigation in both seasons. The respective cob weight in the 2022 season was 237.8, 226.0, and 230.0 g in T1, T2 and T3 under precision drip irrigation compared to 49.8, 68.4 and 63.2 g under water stress. Correspondingly, in 2023 season, T1, T2 and T3 under precision drip had cob weights of 316.4, 310.8, and 300.8 g compared to 260.7, 266.2 and 274.9 g under water stress.
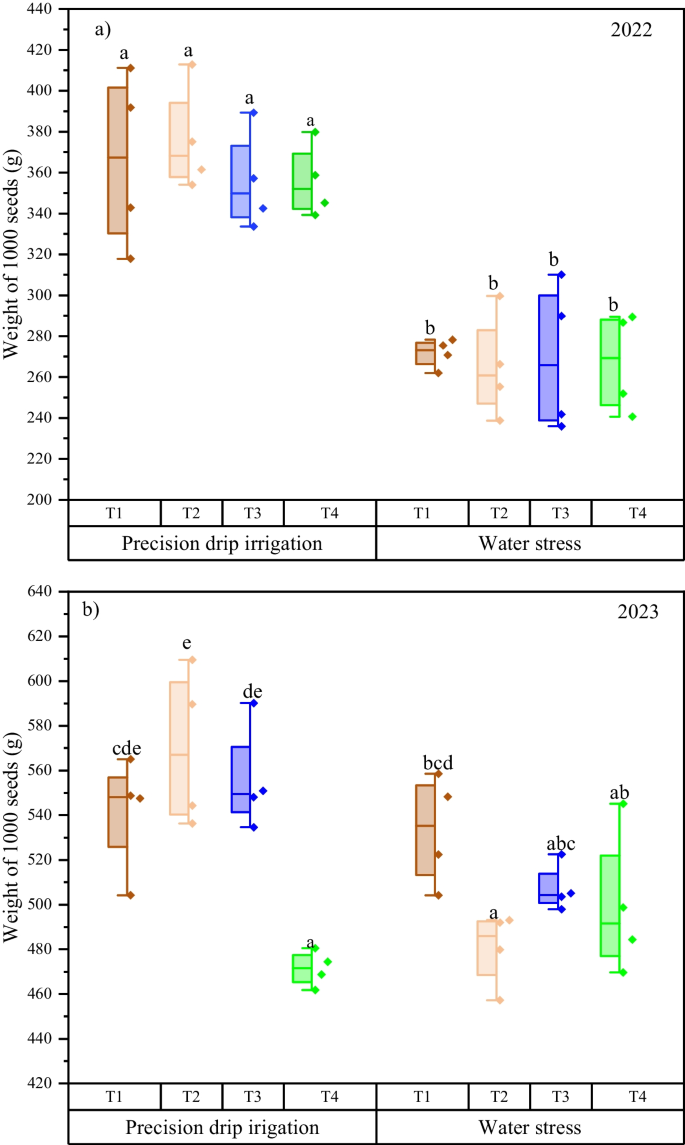
Interactive effects of water management and biostimulant, and micronutrients on the weight of 1000 seeds in 2022 ( a ) and 2023 ( b ) growing seasons. T1 – biostimulant, T2 and T3 – chemical fertilizers containing micronutrients (details in the methodology), T4 – control. Different letters show significant difference between treatments by Tukey test at p < 0.05
3.2.2 Overall Grain Yield (t ha –1 )
Water management, foliar biostimulant and micronutrients application optimized grain yield. In fact, water management significantly ( p < 0.05) improved grain yield in both seasons. Grain yield under precision drip irrigation was 13.3 and 19.4 t ha –1 compared to 3.8 and 17.3 t ha –1 under water stress in 2022 and 2023 growing seasons, respectively. In this case, precision drip irrigation optimized grain yield by 9.5 and 2.1 t ha –1 , respectively. Contrastingly, regardless of the water management regime, biostimulant and micronutrients application significantly ( p < 0.05) optimized grain yield only in 2023 growing season. In 2022 growing season, treatments T1, T2, and T3 had yield between 8.4 and 8.6 t ha –1 compared to 7.1 t ha –1 in the control. In 2023 growing season, grain yields of 18.7, 18.7 and 18.6 t ha –1 were obtained from T1, T2 and T3, respectively compared to 17.5 t ha –1 in the control. Although, the yield of T1, T2 and T3 did not significantly differ from each other, each of these treatments improved yield by 1.0 t ha –1 compared to the control in 2022 season. In 2023 season, grain yield was significantly optimized by 6.9% (1.2 t ha –1 ) under T1 and T2, and 6.3% (1.1 t ha –1 ) under T3 treatments, respectively (Fig. 6 ). The comparative analysis of the efficacy of treatments T1, T2 and T3 between precision drip irrigation and water stress indicated a considerably higher yield under precision drip irrigation compared to water stress. T1 under precision drip irrigation had yield of 14.2 and 20.8 t ha –1 compared to 2.5 and 17.4 t ha –1 under water stress in 2022 and 2023 growing seasons, respectively. Accordingly, T2 under precision drip irrigation had yield of 13.6 and 19.8 t ha –1 compared to 3.7 and 17.6 t ha –1 under water stress in 2022 and 2023 growing seasons, respectively. Figure 7 provides the detailed comparison of grain yield of maize under biostimulant and micronutrient treatments between precision drip irrigation and water stress conditions.
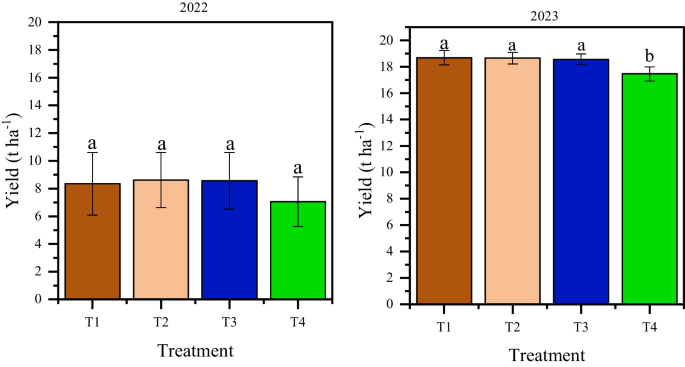
Overall yield response to foliar biostimulant and micronutrient application in 2022 and 2023 seasons. T1 – biostimulant, T2 and T3 – chemical fertilizers containing micronutrients (details in the methodology), T4 – control. Error bar is the standard error. Different letters on the bars in each season show significant difference between treatments by Tukey test at p < 0.05

Grain yield of maize under foliar biostimulant and micronutrient treatments with precision drip irrigation and water stress conditions in 2022 and 2023 growing seasons. IR – Precision drip irrigation. WS – Water stress, T1 – biostimulant, T2 and T3 – chemical fertilizers containing micronutrients (details in the methodology), T4 – control. * and ** means significant differences by Tukey test at p < 0.05 and p < 0.01, respectively. Comparisons were only made where significant differences existed between T1, T2 and T3 between water management regimes
3.2.3 Relationship Between F’, F m ’, Fq ’ , ΦPSII, ETR and Grain Yield
Figure 8 shows the correlation between chlorophyll fluorescence parameters, photochemical yield and grain yield of maize. From the results, F m ’, Fq ’ , ΦPSII and ETR were positively correlated, while F’ negatively correlated with ΦPSII, ETR and grain yield. Further, there was a significant positive correlation between ΦPSII, ETR, and grain yield.
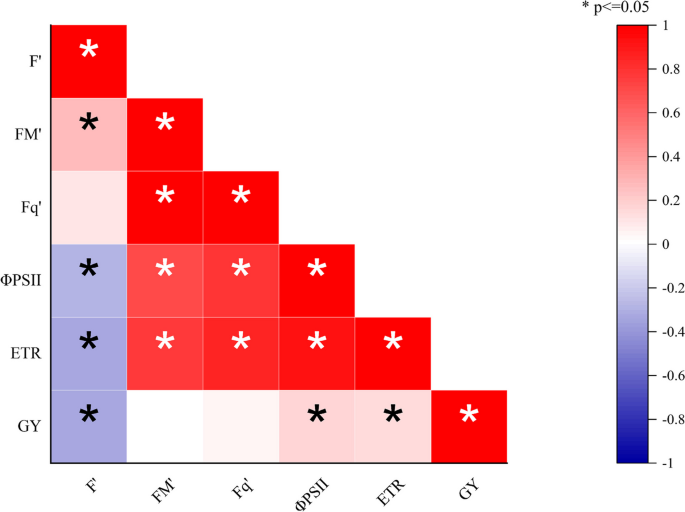
Correlation between chlorophyll fluorescence, quantum efficiency of photosystem II, and grain yield of maize. F ’ (minimal fluorescence), F m ’ ( maximal fluorescence), Fq ’ (fluorescence difference between F m ’ and F ’ , ΦPSII (quantum photosynthetic yield or efficiency of photosystem II), ETR (electron transport rate), GY (Grain yield)
4 Discussion
The photochemical efficiency and yield of maize was optimized by precision drip irrigation in both seasons while the biostimulant and micronutrient significant effects were seasonal. In both seasons, it is only F’ that uniformly decreased at VT stage though higher under water stress, and generally increased at R2 stage. According to dos Reis et al. ( 2019 ), increase in the minimum fluorescence relates to the deleterious effects on photosynthesis, hence reduced excitation energy transfer to the reaction centers from the PSII antenna complex. However, parameters F m ’, Fq ’ , ΦPSII, and ETR depicted opposite patterns in 2022, and similar progressive patterns in 2023 growing season. Although the discrepancy of F m ’, Fq ’ , ΦPSII and ETR was evidenced between the two water regimes, precision drip irrigation maintained optimal effects in both growing seasons. Abiotic stresses including water stress negatively affect chlorophyll biosynthesis, efficiency of photosystems, and ETR, causing reduction in overall photosynthetic efficiency (Sharma et al. 2020 ). Similarly, other reports show diminished photochemical activity and ETR as well as undesirable effects of extreme accumulation of excitation energy on photosynthesis due to water/drought stress (Zivcak et al. 2013 ; Hu et al. 2023 ). In this study, the effects of water stress were significantly evident at the VT and R2 stages because of distinct reduction in the ΦPSII and ETR, which precision drip irrigation ameliorated. In terms of grain yield, precision drip irrigation significantly increased yield in both seasons. However, maize grain yield was generally lower in 2022 compared to 2023, attributed to high heat stress and low precipitation (validated by climate data) between June to August, which were the active months of maize growth. Gombos and Nagy ( 2023 ) conducted an independent study to examine maize growth meteorological conditions at Debrecen in 2022 (similar location as this study) and revealed that the area experienced severe drought exacerbated by high temperatures than before, especially during summer months, making average maize yield to be lower than before. Overall, extreme soil water gradient (high or low) decreases photosynthetic efficiency (Zhao et al. 2019 ) and, in particular, drought stress damages the PSII reaction centers, hence compromising productivity. Generally, in this study, precision drip irrigation ameliorated drought effects on chlorophyll fluorescence, photochemical efficiency, and yield of maize.
Optimization of photochemical yield by the biostimulant and micronutrients was higher under precision drip irrigation compared to water stress. Abidi et al. ( 2023 ) utilized biostimulants from moringa extracts and recorded lower ETR as an indication of decreased response of PSII opening centers to drought, but generally fluorescence parameters were improved under irrigation. Similarly, dos Reis et al. ( 2019 ) revealed that chitosan derivatives positively enhanced photosynthetic activity and electron transport yield in PSII. In this study, biostimulant was rich in nitrogen, organic matter, amino acids and fulvic acid, which improved photosynthetic activity. Studies show that nitrogen increases chlorophyll content and enzymatic activity that promotes efficient functioning of the photosynthetic systems (Nasar et al. 2022 ; Nematpour and Eshghizadeh, 2023 ). Niu et al. ( 2023 ) noted that amino acid glycine betaine regulates growth and development processes in plants. According to Cheng et al. ( 2024 ), optimal application of amino acid containing biostimulants improve productivity and exhibit prospective widespread application to sustain agricultural production. Generally, our results show that the efficacy of the biostimulant and micronutrients improves with supply of adequate moisture through precision drip irrigation. This implies that in the advent of rapidly propagating drought, maximum agronomic efficiency of exogenously applied nutrients is attainable only if adequate moisture is supplied at critical stages of maize growth.
The yield and yield components (cob weight and 1000 seed weight) under treatments were seasonally optimized. Moreover, comparative analysis of yield performance by treatments under precision drip irrigation and water stress indicated a considerably higher yield under precision drip irrigation compared to water stress. Specifically, T1 had higher grain yield in both seasons under precision drip irrigation, suggesting its application efficacy under this water regime. Generally, in both seasons, T1, T2 and T3 reversed the deleterious effects of water stress on grain yield. Reduction in yield due to water stress is attributed to stomatal closure as a responsive mechanism that causes carbon starvation due to lowered CO 2 absorption and transportation (Sharma et al. 2020 ). The results of this study suggest that efficacy of T1, T2 and T3 to enhance maize grain yield and overall productivity is practical under precision irrigation depending on season. However, regardless of the water application regime, the fact that the individual yield of T1, T2 and T3 did not differ from each other (under each water regime), but differed compared to the control, suggests the need to test the effect of combined foliar application of these treatments to ascertain the existence of synergistic effects. Based on soil analysis results, nitrogen soil content was inadequate. This implies single application of T1 did not meet maize nitrogen demand for optimum productivity. This suggests two options: first, to maintain single T1 application but boost nitrogen supply to maize through increased soil application. However, this may not be sustainable because of potential effects of nitrogen pollution. Secondly, to have multiple applications of T1 at different phenophases. These necessitate further investigation to ascertain exact times of application versus precision drip irrigation regimes. In other words, the productivity potential of the biostimulant was not attained. Crop response to biostimulants depends on biostimulant formulation and utilization approach, climate conditions (Długosz et al. 2020 ), plant factors (Berta et al. 2014 ), among other factors. Similarly, the fact that zinc was adequate in the soil suggests the need to test the efficacy of T2 in zinc deficient soils (locations) under varying drip irrigation regimes. Hussain et al. ( 2020 ) reported similar yield of maize in potassium and zinc fertilized plots with well-irrigated plots without potassium and zinc fertilization, and the authors emphasized the need for evaluation of irrigation scheduling. However, Idrees et al. ( 2024 ) noted that zinc supplementation enhanced yield components compared to non-zinc treatment both under watered and water stress conditions. Similarly, Elshamly et al. ( 2024 ) revealed that with 75% irrigation, zinc application enhanced optimum dry matter accumulation and economic yield. Zinc is a critical micronutrient that regulates metabolic processes and enhances photosynthetic carbon assimilation by maize under severe abiotic stress (Sun et al. 2021 ; Ghani et al. 2022 ; Idrees et al. 2024 ).
The correlation analysis revealed a significant positive nexus between ETR, ΦPSII and grain yield. This is an indication that ΦPSII and ETR are better indicators of the potential effect of water management, foliar biostimulant and micronutrient application on maize productivity. Jin et al. ( 2023 ) reported a positive correlation between chlorophyll fluorescence and photosynthesis, with a substantial increase in water stress. However, variation in the results of chlorophyll and photochemical parameters at different stages of maize growth indicates the possibility of using these parameters to detect and ameliorate deleterious water stress effects on maize yield at early stages. In other words, this will make utilization of agronomic interventions sustainable since early remediation measures enhance attainment of planned yield.
5 Conclusion
Fertilization and precision water supply remain the principal impetuses to the achievement of sustainable maize productivity. In this study, precision drip irrigation, foliar application of the biostimulant and micronutrients optimized maximal fluorescence, photosynthetic efficiency and electron transport rate. Although precision drip irrigation significantly optimized yield and yield components in both seasons, biostimulant and micronutrient effects were seasonally conspicuous regardless of irrigation. Comparative performance of the biostimulant and micronutrients under water management regimes indicated higher yield under precision irrigation compared to water stress. However, since the soils in the experimental site were deficient in nitrogen and adequate in zinc, it suggests several research gaps: (i) a study tailored to 2–3 application times at critical stages of maize under season specific precision irrigation, (ii) need to test the effect of combined foliar application of T1 (non-microbial biostimulant from plant origin), T2 (zinc based chemical fertilizer), and T3 (boron and molybdenum based chemical fertilizer) to ascertain the existence of synergistic effects, and (iii) need to test the efficacy of T2 in soil types (locations) deficient in zinc under precision drip irrigation. Overall, precision drip irrigation, foliar application of biostimulant and micronutrients demonstrated their capacity to optimize photosynthetic efficiency, yield and yield components of maize.
Data Availability
All the data are presented in the article.
Abbott LK, Macdonald LM, Wong MTF, Webb MJ, Jenkins SN, Farrell M (2018) Potential roles of biological amendments for profitable grain production – A review. Agric Ecosyst Environ 256:34–50. https://doi.org/10.1016/j.agee.2017.12.021
Article Google Scholar
Abidi W, Akrimi R, Gouiaa M (2023) Mitigating drought stress by plant-derived biostimulant in Arbequina olive ( Oleaeuropeae L.) cultivar conducted in super high density. Acta Physiol Plant 45:132. https://doi.org/10.1007/s11738-023-03613-9
Article CAS Google Scholar
Alloway BJ (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31:537–548. https://doi.org/10.1007/s10653-009-9255-4
Article CAS PubMed Google Scholar
Antal J, Buzás I, Debreczeni B, Fekete A, Nagy M, Patócs I (1987) New fertilization guidelines MÉM NAK. Budapest, pp 1–65
Arikan B, Ozfidan-Konakci C, Alp FN, Zengin G, Yildiztugay E (2022) Rosmarinic acid and hesperidin regulate gas exchange, chlorophyll fluorescence, antioxidant system and the fatty acid biosynthesis-related gene expression in Arabidopsis thaliana under heat stress. Phytochem 198:113157. https://doi.org/10.1016/j.phytochem.2022.113157
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113. https://doi.org/10.1146/annurev.arplant.59.032607.092759
Berta G, Copetta A, Gamalero E, Bona E, Cesaro P, Scarafoni A, D’Agostino G (2014) Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 24:161–170. https://doi.org/10.1007/s00572-013-0523-x
Article PubMed Google Scholar
Bojtor C, Illés Á, Mousavi SMN, Széles A, Tóth B, Nagy J, Marton CL (2021) Evaluation of the nutrient composition of maize in different NPK fertilizer levels based on multivariate method analysis. Inter J Agron 2021:5537549. https://doi.org/10.1155/2021/5537549
Bresson J, Vasseur F, Dauzat M, Koch G, Vile D (2015) Quantifying spatial heterogeneity of chlorophyll fluorescence during plant growth and in response to water stress. Plant Methods 11:23. https://doi.org/10.1186/s13007-015-0067-5
Article CAS PubMed PubMed Central Google Scholar
Cendrero-Mateo MP, Moran MS, Papuga SA, Thorp KR, Alonso L, Moreno J, Ponce-Campos G, Rascher U, Wang G (2016) Plant chlorophyll fluorescence: active and passive measurements at canopy and leaf scales with different nitrogen treatments. J Exp Bot 67:275–286. https://doi.org/10.1093/jxb/erv456
Chen Y, Liu L, Guo Q, Zhu Z, Zhang L (2016) Effects of different water management options and fertilizer supply on photosynthesis, fluorescence parameters and water use efficiency of Prunella vulgaris seedlings. Biol Res 49:12. https://doi.org/10.1186/s40659-016-0069-4
Chen X, Mo X, Hu S, Liu S (2019) Relationship between fluorescence yield and photochemical yield under water stress and intermediate light conditions. J Exp Bot 70:301–313. https://doi.org/10.1093/jxb/ery341
Chen C, Lin K, Chang Y, Chang Y (2023) Application of water-saving irrigation and biostimulants on the agronomic performance of maize ( Zea mays ). Process Saf Environ Prot 77:1377–1386. https://doi.org/10.1016/j.psep.2023.08.008
Cheng J, Cheng J, Sun R, Cao S, Wang X, Yang H (2024) Foliar application of amino acid biostimulants increased growth and antioxidant activity of Epipremnum aureum . Cogent Food Agric 10:1–10. https://doi.org/10.1080/23311932.2024.2321680
Długosz J, Piotrowska-Długosz A, Kotwica K, Przybyszewska E (2020) Application of multi-component conditioner with clinoptilolite and Ascophyllum nodosum extract for improving soil properties and Zea mays L. growth and yield. Agron 10:2005. https://doi.org/10.3390/agronomy10122005
Dogru A (2021) Effects of heat stress on photosystem II activity and antioxidant enzymes in two maize cultivars. Planta 253:85. https://doi.org/10.1007/s00425-021-03611-6
dos Reis CO, Magalhães PC, Avila RG, Almeida GL, Rabelo MV, Carvalho TD, Cabral FD, Karam D, Souza CT (2019) Action of N -Succinyl and N,O -Dicarboxymethyl chitosan derivatives on chlorophyll photosynthesis and fluorescence in drought-sensitive maize. J Plant Growth Regul 38:619–630. https://doi.org/10.1007/s00344-018-9877-9
Elshamly AMS, Iqbal R, Ali B, Ahmed I, Akram MI, Ali S, Ditta A, Elshikh ÇIGF, Mustafa MS, Hamed AEMA HM (2024) Zinc and amino acids improve the growth, physiological, and biochemical attributes of corn under different irrigation levels. Rhizosphere 29:100820. https://doi.org/10.1016/j.rhisph.2023.100820
Flexas J, Escalona JM, Medrano H (2002) Water stress induces different levels of photosynthesis and electron transport rate regulation in grapevines. Plant Cell Environ 22:39–48. https://doi.org/10.1046/j.1365-3040.1999.00371.x
Ge TD, Sui GF, Nie S, Sun NB, Xiao H, Tong LC (2010) Differential responses of yield and selected nutritional compositions to drought stress in summer maize grains. J Plant Nutr 33:1811–1818. https://doi.org/10.1080/01904167.2010.503829
Ghani MI, Saleem S, Rather SA, Rehmani MS, Alamri S, Rajput VD, Kalaji HM, Sial TA, Liu M (2022) Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 289:133202. https://doi.org/10.1016/j.chemosphere.2021.133202
Gombos B, Nagy G (2023) Meteorological conditions of maize growing experiments on agricultural campus of the University of Debrecen in the growing season 2022. Crop Prod 72:5–19
Google Scholar
Guo Y, Tan J (2015) Recent advances in the application of Chlorophyll a fluorescence from Photosystem II. Photochem Photobio 91:1–14. https://doi.org/10.1111/php.12362
Hayat SH, Rehman A, Farooq A, Naveed M, Ali HM, Hussain M (2023) Boron seed coating combined with seed inoculation with boron tolerant bacteria ( Bacillus sp. MN-54) and maize stalk biochar improved growth and productivity of maize ( Zea mays L.) on saline soil. Heliyon 9:e22075. https://doi.org/10.1016/j.heliyon.2023.e22075
Hazrati S, Tahmasebi-Sarvestani Z, Modarres-Sanavy SAM, Mokhtassi-Bidgoli A, Nicola S (2016) Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol Biochem 106:141–148. https://doi.org/10.1016/j.plaphy.2016.04.046
Hu F, Zhang Y, Guo J (2023) Effects of drought stress on photosynthetic physiological characteristics, leaf microstructure, and related gene expression of yellow horn. Plant Signal Behav 18(1):2215025. https://doi.org/10.1080/15592324.2023.2215025
Hussain S, Maqsood M, Ijaz M, Ul-Allah S, Sattar A, Sher A, Nawaz A (2020) Combined application of potassium and zinc improves water relations, stay green, irrigation water use efficiency, and grain quality of maize under drought stress. J Plant Nutri 43:2214–2225. https://doi.org/10.1080/01904167.2020.1765181
Idrees K, Aziz A, Naeem M, Azhar FM, Farooq S, Hussain M (2024) Combined application of zinc and silicon improved growth, gas exchange traits, and productivity of maize ( Zea mays L.) under water stress. Silicon 16:831–841. https://doi.org/10.1007/s12633-023-02732-9
Janka E, Korner O, Rosenqvist E, Ottosen C (2015) Using the quantum yields of photosystem II and the rate of net photosynthesis to monitor high irradiance and temperature stress in chrysanthemum ( Dendranthema Grandiflora) . Plant Physiol Biochem 90:14e22. https://doi.org/10.1016/j.plaphy.2015.02.019
Janušauskaite D, Feiziene D (2011) Chlorophyll fluorescence characteristics throughout spring triticale development stages as affected by fertilization. Acta Agric Scand B 62:7–15. https://doi.org/10.1080/09064710.2011.560122
Jin EJ, Yoon JH, Lee H, Bae EJ, Yong SH, Choi MS (2023) Evaluation of drought stress level in Sargent’s cherry ( Prunus Sargentii Rehder) using photosynthesis and chlorophyll fluorescence parameters and proline content analysis. Peer J 11:e15954. https://doi.org/10.7717/peerj.15954
Killi D, Bussotti F, Raschi A, Haworth M (2017) Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiol Plant 159:130–147. https://doi.org/10.1111/ppl.12490
Killi D, Raschi A, Bussotti F (2020) Lipid peroxidation and chlorophyll fluorescence of photosystem II performance during drought and heat stress is associated with the antioxidant capacities of C3 sunflower and c4 maize varieties. Int J Mol Sci 21:4846. https://doi.org/10.3390/ijms21144846
Klofac D, Antosovsky J, Skarpa P (2023) Effect of zinc foliar fertilization alone and combined with trehalose on maize ( Zea may s L.) growth under the drought. Plants 12:2539. https://doi.org/10.3390/plants12132539
Kovács AB, Kincses I, Vágó I, Loch J, Filep T (2009) Effect of application of nitrogen and different nitrogen-sulfur ratios on the quality and quantity of mustard seed. Commun Soil Sci Anal 40:453–461. https://doi.org/10.1080/00103620802694399
Kumar S, Narjary B, Paudayal K, Yadav RK, Kamra SK (2024) Assessing zero-till direct seeding at variable water stress levels compared to traditional puddled transplanting of rice under groundwater-fed irrigation systems in north-west India. Irrig Drain 73:928–943. https://doi.org/10.1002/ird.2930
Li G, Zhang ZS, Gao HY, Liu P, Dong ST, Zhang JW, Zhao B (2012) Effects of nitrogen on photosynthetic characteristics of leaves from two different stay-green corn ( Zea mays L.) varieties at the grain-filling stage. Can J Plant Sci 92:671–680. https://doi.org/10.4141/cjps2012-039
Loriaux SD, Avenson TJ, Welles JM, McDermitt DK, Eckles RD, Riensche B, Genty BSD (2013) Closing in on maximum yield of chlorophyll fluorescence using a single multiphase flash of sub-saturating intensity. Plant Cell Environ 36:1755–1770. https://doi.org/10.1111/pce.12115
Ma D, Sun D, Wang C, Ding H, Qin H, Hou J, Huang X, Xie Y, Guo T (2017) Physiological responses and yield of wheat plants in zinc-mediated alleviation of drought stress. Front Plant Sci 8:860. https://doi.org/10.3389/fpls.2017.00860
Article PubMed PubMed Central Google Scholar
Nasar J, Wang G–Y, Ahmad S, Muhammad I, Zeeshan M, Gitari H, Adnan M, Fahad S, Khalid MHB, Zhou X–B, Abdelsalam NR, Ahmed GA, Hasan ME (2022) Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front Plant Sci 13:988055. https://doi.org/10.3389/fpls.2022.988055
Nematpour A, Eshghizadeh HR (2023) Biochemical responses of sorghum and maize to the impacts of different levels of water deficit and nitrogen supply. Cereal Res Commun. https://doi.org/10.1007/s42976-023-00411-4
Niu T, Zhang J, Li J, Gao X, Ma H, Gao Y, Chang Y, Xie J (2023) Effects of exogenous glycine betaine and cycloleucine on photosynthetic capacity, amino acid composition, and hormone metabolism in Solanum melongena L. Sci Rep 13:7626. https://doi.org/10.1038/s41598-023-34509-w
Noulas C, Tziouvalekas M, Karyotis T (2018) Zinc in soils, water and food crops. J Trace Elem Med Biol 49:252–260. https://doi.org/10.1016/j.jtemb.2018.02.009
Ocwa A, Harsanyi E, Széles A, Holb IJ, Szabó S, Rátonyi T, Mohammed S (2023) A bibliographic review of climate change and fertilization as the main drivers of maize yield: implications for food security. Agric Food Secur 12:14. https://doi.org/10.1186/s40066-023-00419-3
Ocwa A, Mohammed S, Mousavi SMN, Illés Á, Bojtor C, Ragán P, Rátonyi T, Harsányi H (2024) Maize grain yield and quality improvement through biostimulant application: a systematic review. J Soil Sci Plant Nutr 24:1609–1649. https://doi.org/10.1007/s42729-024-01687-z
Prasanna R, Hossain F, Saxena G, Singh B, Kanchan A, Simranjit K, Ramakrishnan B, Ranjan K, Muthusamy V, Shivay YS (2021) Analyses of genetic variability and genotype x cyanobacteria interactions in biofortified maize ( Zea mays L.) for their responses to plant growth and physiological attributes. Eur J Agron 130:126343. https://doi.org/10.1016/j.eja.2021.126343
Rácz D, Szőke L, Tóth B, Kovács B, Horváth É, Zagyi P, Duzs L, Széles A (2021) Examination of the productivity and physiological responses of maize ( Zea mays L.) to Nitrapyrin and foliar fertilizer treatments. Plants 10:2426. https://doi.org/10.3390/plants10112426
Rehaman A, Fatma M, Jan AT, Shah AA, Asgher M, Khan NA (2023) Co-application of nitric oxide and vermicompost improves photosynthetic functions, antioxidants, and nitrogen metabolism in maize ( Zea mays L.) grown under drought stress. J Plant Growth Regul 42:3888–3907. https://doi.org/10.1007/s00344-022-10854-4
Sadeghzadeh B (2013) A review of zinc nutrition and plant breeding. J Soil Sci Plant Nutr 13:6907–6927. https://doi.org/10.4067/S0718-95162013005000072
Sharma A, Kumar V, Shahzad B, Ramakrishnan M, Sidhu SPG, Bali SA, Handa N et al (2020) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 39:509–531. https://doi.org/10.1007/s00344-019-10018-x
Sible CN, Seebauer JR, Below FE (2021) Plant biostimulants: a categorical review, their implications for row crop production, and relation to soil health indicators. Agron 11:1297. https://doi.org/10.3390/agronomy11071297
Simkó A, Gáspár SG, Kiss L, Makleit P, Veres S (2020) Evaluation of nitrogen nutrition in diminishing water deficiency at different growth stages of maize by chlorophyll fluorescence parameters. Plants 9:676. https://doi.org/10.3390/plants9060676
Sinsawat V, Leipner J, Stamp P, Fracheboud Y (2004) Effect of heat stress on the photosynthetic apparatus in maize ( Zea mays L.) grown at control or high temperature. J Exp Bot 52:123–129. https://doi.org/10.1016/j.envexpbot.2004.01.010
Ssemugenze B, Ocwa A, Bojtor C, Illés Á, Esimu J, Nagy J (2024) Impact of research on maize production challenges in Hungary. Heliyon 10:e26099. https://doi.org/10.1016/j.heliyon.2024.e26099
Sun L, Song F, Zhu X, Liu S, Liu F, Wang Y, Li X (2021) Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch Agron Soil Sci 67:245–259. https://doi.org/10.1080/03650340.2020.1723003
Toth B, Moloi MJ, Mousavi SMN, Illés Á, Bojtor C, Szoke L, Nagy J (2022) The evaluation of the effects of Zn, and amino acid-containing foliar fertilizers on the physiological and biochemical responses of a Hungarian fodder corn hybrid. Agron 12:1523. https://doi.org/10.3390/agronomy12071523
Trivedi K, Vijay Anand KG, Kubavat D, Patidar R, Ghosh A (2018) Drought alleviatory potential of Kappaphycus seaweed extract and the role of the quaternary ammonium compounds as its constituents towards imparting drought tolerance in Zea mays L. J Appl Phycol 30:2001–2015. https://doi.org/10.1007/s10811-017-1375-0
Tubuxin B, Rahimzadeh-Bajgiran P, Ginnan Y, Hosoi F, Omasa K (2015) Estimating chlorophyll content and photochemical yield of photosystem II (ΦPSII) using solar-induced chlorophyll fluorescence measurements at different growing stages of attached leaves. J Exp Bot 66:5595–5603. https://doi.org/10.1093/jxb/erv272
Wang H, Jin Y (2005) Photosynthetic rate, chlorophyll fluorescence parameters, and lipid peroxidation of maize leaves as affected by zinc deficiency. Photosynthetica 43:591–596. https://doi.org/10.1007/s11099-005-0092-0
Waraich EA, Ahmad R, Saifullah, Ashraf MY, Ehsanullah (2011) Role of mineral nutrition in alleviation of drought stress in plants. Aust J Crop Sci 5:764–777
CAS Google Scholar
Yin X, Sun Z, Struik PC, Van Der PuttenPEL,VanIeperen W, Harbinson J (2011) Using a biochemical C4 photosynthesis model and combined gas exchange and chlorophyll fluorescence measurements to estimate bundle-sheath conductance of maize leaves differing in age and nitrogen content. Plant Cell Environ 34:2183–2199. https://doi.org/10.1111/j.1365-3040.2011.02414.x
Zampieri M, Ceglar A, Dentener F, Dosio A, Naumann G, van den Berg M, Toreti A (2019) When will current climate extremes affecting maize production become the norm? Earth’s Futur 7:113–122. https://doi.org/10.1029/2018EF000995
Zhang L, Yan M, Li H, Ren Y, Siddique HMK, Chen Y, Zhang S (2020) Effects of zinc fertilizer on maize yield and water-use efficiency under different soil water conditions. Field Crops Res 248:107718. https://doi.org/10.1016/j.fcr.2020.107718
Zhao J, Lang Y, Zhang S, Zhao Q, Zhang C, Xia J (2019) Photosynthetic characteristics and chlorophyll a fluorescence transient in Lonicera japonica under drought stress. Acta Physiol Plant 41:124. https://doi.org/10.1007/s11738-019-2912-z
Zivcak M, Brestic M, Balatova Z, Drevenakova P, Olsovska K, Kalaji HM, Yang X, Allakhverdiev SI (2013) Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth Res 117:529–546. https://doi.org/10.1007/s11120-013-9885-3
Download references
Open access funding provided by University of Debrecen. Project no. TKP2021-NKTA-32 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme and supported by the EKÖP-24-4 University Research Scholarship Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. This paper was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00068/23/4).
Author information
Authors and affiliations.
Institute of Land Use, Engineering and Precision Farming Technology, Faculty of Agricultural and Food Sciences and Environmental Management, University of Debrecen, Böszörményi út 138, Debrecen, 4032, Hungary
Akasairi Ocwa, Csaba Bojtor, Árpád Illés, Brian Ssemugenze, Ibtissem Balaout, Tamás Rátonyi, Adrienn Széles & Endre Harsányi
Kálmán Kerpely Doctoral School, University of Debrecen, 138 Böszörményi street, Debrecen, 4032, Hungary
Akasairi Ocwa, Brian Ssemugenze & Ibtissem Balaout
Institutes for Agricultural Research and Educational Farm, University of Debrecen, Böszörményi 138, Debrecen, 4032, Hungary
Endre Harsányi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Akasairi Ocwa .
Ethics declarations
Conflict of interest.
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Ocwa, A., Bojtor, C., Illés, Á. et al. Precision drip Irrigation System and Foliar Application of Biostimulant and Fertilizers Containing Micronutrients Optimize Photochemical Efficiency and Grain Yield of Maize ( Zea mays L). J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-02074-4
Download citation
Received : 15 April 2024
Accepted : 08 October 2024
Published : 30 October 2024
DOI : https://doi.org/10.1007/s42729-024-02074-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biostimulant
- Micronutrients
- Precision drip irrigation
- Photochemistry
- Water stress
- Find a journal
- Publish with us
- Track your research
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
Economic activity and social change in the UK, real-time indicators: 31 October 2024
Early experimental data and analysis on economic activity and social change in the UK. These real-time indicators are created using rapid response surveys, novel data sources and experimental methods.
https://www.ons.gov.uk/releases/economicactivityandsocialchangeintheukrealtimeindicators31october2024
Official statistics are produced impartially and free from political influence.
Updates to this page
Sign up for emails or print this page, is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
“It’s a symbol of creativity”: Les Paul’s experimental Klunker helped pave the way for his later innovations – now it’s been revived as a meticulous one-off replica by Les Paul's personal tech
Renowned luthier Tom Doyle hopes it will deepen the public’s understanding of the guitar’s cultural significance

Esteemed luthier Tom Doyle – who once worked as Les Paul's personal tech – has unveiled a “meticulously crafted historical replica” of the Klunker guitar – an instrument entwined in the story of the Les Paul guitar.
Its arrival was foreshadowed over “years of dedicated research” to offer a new, tangible insight into the early development of Les Paul’s namesake and change-making electric guitar .
The pre-cursor to the Les Paul, the Klunker and its early stablemates strummed before its successor could shred. Created in the 1940s, three iterations of the Klunker – alongside "the Log" – were devised as early testbeds for Paul's experimentation with amplification and pickup design.
Doors were installed in its rear to allow easy access to its work-in-progress wiring configurations, which included homemade and hand-wound pickups.
The Klunkers, ultimately, sat at the core of the new sound craved by the hugely innovative guitarist and luthier, who once said he would only stop playing the instruments when Gibson turned his grand design of a solid-body electric guitar into a reality.
In an intriguing 'What if?' moment, it turns out Leo Fender saw the appeal of Les Paul's design long before Gibson did, meaning the Les Paul could well have been a Fender model if it weren't for its creator's determination to have the Gibson name across its headstock.
Indeed, Gibson only agreed to do so after Fender had found success with its own design, the Broadcaster.
Get The Pick Newsletter
All the latest guitar news, interviews, lessons, reviews, deals and more, direct to your inbox!
Fast forward to 2024, the Les Paul is still adored after generations of riff-slinging, and now Doyle has launched a Klunker replica as “a tribute to the guitar’s historical significance and Les Paul’s inventive spirit”.

“The Klunker is not just a piece of musical equipment; it’s a symbol of innovation and creativity,” he goes on. “By recreating this historic guitar, we’re able to shed light on the origins of the Gibson Les Paul and the vision that drove its development.
“This replica is a testament to Les Paul's influence and a chance for new generations to understand the origins of one of the most important guitars in history.”
The original guitar was made from maple, rosewood and walnut, with featured mother-of-pearl block inlays and plastic parts. Doyle has dedicated his time into replicating every facet of the original Klunker spirit with his build.
With the guitar now unveiled, Doyle intends to take it on tour, where he will conduct workshops and discussions focused on the Klunker’s history, its cultural significance, and the process of its replica’s creation.
Thank you for reading 5 articles this month**
Join now for unlimited access
US pricing $3.99 per month or $39.00 per year
UK pricing £2.99 per month or £29.00 per year
Europe pricing €3.49 per month or €34.00 per year
*Read 5 free articles per month without a subscription
Prices from £2.99/$3.99/€3.49
A freelance writer with a penchant for music that gets weird, Phil is a regular contributor to Prog , Guitar World , and Total Guitar magazines and is especially keen on shining a light on unknown artists. Outside of the journalism realm, you can find him writing angular riffs in progressive metal band, Prognosis , in which he slings an 8-string Strandberg Boden Original, churning that low string through a variety of tunings. He's also a published author and is currently penning his debut novel which chucks fantasy, mythology and humanity into a great big melting pot.
“Dependability and durability baked in”: Cort takes a leaf out of Strandberg’s book and begins its True Temperament experiments with the KX700 TT
“I would pick this over a ’60s or ’70s model. It’s the perfect Telecaster in my eyes”: Tele obsessive John 5 provides the ultimate test for Fender’s new American Ultra II series
“I called Supro and said, 'Hey I’m really digging this amp. Is there any way we can just give it a little more horsepower?'” Blues rock ace Tyler Bryant supercharges Supro’s flagship Black Magick combo for his new signature amp
Most Popular
- 2 “Dependability and durability baked in”: Cort takes a leaf out of Strandberg’s book and begins its True Temperament experiments with the KX700 TT
- 3 “I ended up playing things I never have before”: John Mayer busts out his Charvel and an ultra-rare ESP as he breaks new ground during A-list R&B guest solo
- 4 “I went there with the intention of buying a Gretsch Jet Firebird. He said, ‘Forget the Gretsch. That’s a toy compared to the 335’”: Ritchie Blackmore recalls buying his first Gibson from amp pioneer Jim Marshall's music store
- 5 “I would pick this over a ’60s or ’70s model. It’s the perfect Telecaster in my eyes”: Tele obsessive John 5 provides the ultimate test for Fender’s new American Ultra II series
We've detected unusual activity from your computer network
To continue, please click the box below to let us know you're not a robot.
Why did this happen?
Please make sure your browser supports JavaScript and cookies and that you are not blocking them from loading. For more information you can review our Terms of Service and Cookie Policy .
For inquiries related to this message please contact our support team and provide the reference ID below.

IMAGES
VIDEO
COMMENTS
Determine the Theoretical Yield: Use stoichiometry to find the theoretical yield, ensuring it is in the same units as the actual yield. Measure the Actual Yield: Obtain this from your experimental data. Apply the Percent Yield Formula: Insert your actual and theoretical yields into the percent yield formula to find the efficiency of your reaction.
Calculating Experimental Yields. 1. Ensure you have a correctly balanced equation for the reaction performed. 2. Determine how many moles of each species were used in the reaction. 3. Determine which species is the limiting reagent, remembering to use the reaction stoichiometry. 4.
The theoretical yield of a reaction is the amount of product you would get if you use up all of the limiting reagent, and if there is no loss, e.g. by degradation of reactant or product or by formation of byproducts. The experimental yield is the actual amount of product you obtain when performing a given experiment. The percentage yield is a ...
DEFINITIONS: theoretical yield: the quantity of product theoretically obtainable from a given quantity of reactant in a chemical reaction. actual yield: the experimental quantity of product obtained from a chemical reaction. percent yield: the ratio of actual yield to the theoretical yield, multiplied by 100%. Yields.
Percent Yield. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical equation, is called the theoretical yield of the reaction. In practice, the amount of product obtained is called the actual yield, and it is often less than the theoretical yield for a number of reasons.
The actual yield is the amount that was actually made, which was 65.2 g of Zn(NO 3) 2. To calculate the percent yield, we take the actual yield and divide it by the theoretical yield and multiply by 100: 65.2 g Zn(NO 3) 2 88.3 Zn(NO 3) 2 × 100 % = 73.8 % The worker achieved almost three-fourths of the possible yield. Test Yourself
In chemistry, yield, also known as reaction yield or chemical yield, refers to the amount of product obtained in a chemical reaction. [1] Yield is one of the primary factors that scientists must consider in organic and inorganic chemical synthesis processes. [2] In chemical reaction engineering, "yield", "conversion" and "selectivity" are terms used to describe ratios of how much of a reactant ...
Actual Yield Definition. Actual yield is the amount of product you experimentally obtain from a chemical reaction. In contrast, theoretical yield is the amount of product you obtain if all of the reactant converts to product. Actual yield is an empirical value that you measure in the lab, while theoretical yield is a calculated value.
Lesson 4: Stoichiometry. Stoichiometry. Worked example: Calculating amounts of reactants and products. Limiting reactant and reaction yields. Worked example: Calculating the amount of product formed from a limiting reactant. Worked example: Relating reaction stoichiometry and the ideal gas law. Stoichiometry.
Our percent yield calculator will help you to understand how to calculate the percent yield, as well as teach you the percent yield formula and the percent yield definition.. Finding the yield is an integral part of any kind of synthetic lab work as the percent yield equation turns your experimental yields into a representation of how successfully you carried out your reaction.
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated, multiplied by 100%. % yield = actual yield theoretical yield ⋅ 100%. So, let's say you want to do an experiment in the lab. You want to measure how much water is produced when 12.0 g of glucose (C6H 12O6) is burned with enough ...
All you need to do is plug the values into the formula: percent yield = actual yield / theoretical yield x 100%. percent yield = 15 g / 19 g x 100%. percent yield = 79%. Usually, you have to calculate the theoretical yield based on the balanced equation. In this equation, the reactant and the product have a 1:1 mole ratio, so if you know the ...
This spacing is crucial for isolating the effects of specific experimental factors being studied. For physiological, biochemical, and seed quality traits, 30 replications were used to ensure sufficient precision in capturing biological variation. ... The seed yield was then standardized to a moisture content of 12% and expressed in kilograms ...
The word semantics, in robotics and AI, has no canonical definition. It usually serves to denote additional data provided to autonomous agents to aid HRI. Most researchers seem, implicitly, to understand that such data cannot simply be extracted from environmental data. I try to make explicit why this is so and argue that so-called semantics are best understood as data comprised of conventions ...
In three steps, the mass-mass calculation is. Thus, the theoretical yield is 88.3 g of Zn (NO 3) 2. The actual yield is the amount that was actually made, which was 65.2 g of Zn (NO 3) 2. To calculate the percent yield, we take the actual yield and divide it by the theoretical yield and multiply by 100: The worker achieved almost three-fourths ...
The grain yield (GY) was calculated by taking the total weight of ears from each experimental unit, discounting the corncob percentage (corncob weight/ear weight) estimated from a 5-ear sample, and involving an additional adjustment to 14% moisture, the result was reported in t ha −1. Other traits registered were the number of grains in 10 g ...
This study evaluated the effect of precision drip irrigation, biostimulant, and micronutrients application on photochemical efficiency and yield of maize.An experiment laid in a randomized complete block design with irrigation and water stress was established in 2022 and 2023 growing seasons at the experimental area of the University of Debrecen.
Early experimental data and analysis on economic activity and social change in the UK. These real-time indicators are created using rapid response surveys, novel data sources and experimental methods.
(Image credit: Tom Doyle) "The Klunker is not just a piece of musical equipment; it's a symbol of innovation and creativity," he goes on. "By recreating this historic guitar, we're able to shed light on the origins of the Gibson Les Paul and the vision that drove its development. "This replica is a testament to Les Paul's influence and a chance for new generations to understand the ...
An elderly patient died in a clinical study of Roche Holding AG's experimental treatment for Alzheimer's disease, prompting changes to the trial design, although the company said overall ...